Abstract
Purpose
The purpose of this study was to undertake dual assessment of tumour blood flow and glucose metabolism in non-small cell lung cancer (NSCLC) using contrast-enhanced computed tomography (CE-CT) and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) in order to assess how the relationships between these parameters vary with tumour size and stage.
Methods
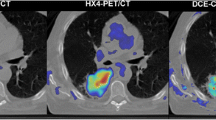
Tumour blood flow and glucose metabolism were assessed in 18 NSCLCs using quantitative CE-CT and FDG-PET respectively. Contrast enhancement and FDG uptake were both normalised to injected dose and patient weight to yield correspondingly the standardised perfusion value (SPV) and standardised uptake value (SUV). Tumour area was measured from conventional CT images.
Results
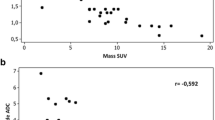
The ratio of SUV to SVP and the metabolic–flow difference (SUV–SVP) correlated with tumour size (r=0.56, p=0.015 and r=0.60 and p=0.008 respectively). A metabolic–flow difference of greater than 4 was more common amongst tumours of stages III and IV (odds ratio 10.5; 95% confidence limits 0.24–32.1). A significant correlation between SUV and SPV was found only for tumours smaller than 4.5 cm2 (r=0.85, p=0.03).
Conclusion
Blood flow–metabolic relationships are not consistent in NSCLC but depend upon tumour size and stage. Quantitative CE-CT as an adjunct to an FDG study undertaken using integrated PET-CT offers an efficient way to augment the assessment of tumour biology with possible future application as part of clinical care.



Similar content being viewed by others
References
Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914–24
Swensen SJ, Viggiano RW, Midthun DE, Muller NL, Sherrick A, Yamashita K, et al. Lung nodule enhancement at CT: multicenter study. Radiology 2000;214:73–80
Pastorino U, Bellomi M, Landoni C, De Fiori E, Arnaldi P, Picchio M, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet 2003;362:593–7
Swensen SJ, Brown LR, Colby TV, Weaver AL, Midthun DE. Lung nodule enhancement at CT: prospective findings. Radiology 1996;201:447–55
Tateishi U, Nishihara H, Watanabe S, Morikawa T, Abe K, Miyasaka K. Tumor angiogenesis and dynamic CT in lung adenocarcinoma: radiologic-pathologic correlation. J Comput Assist Tomogr 2001;25:23–7
Yi CA, Lee KS, Kim EA, Han J, Kim H, Kwon OJ, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004;233:191–9
Comber LA, Keith CJ, Griffiths M, Miles KA. Solitary pulmonary nodules: impact of quantitative contrast-enhanced CT on the cost-effectiveness of FDG-PET. Clin Radiol 2003;58:706–11
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 1999;35:1773–82
Mac Manus MP, Hicks R J, Matthews JP, McKenzie A, Rischin D, Salminen EK, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol 2003;21:1285–92
Miller JC, Pien HH, Sahani D, Sorensen G, Thrall JH. Imaging angiogenesis: applications and potential for drug development. J Natl Cancer Inst 2005;97:172–87
Kiessling F, Boese J, Corvinus C, Ederle JR, Zuna I, Schoenberg SO, et al. Perfusion CT in patients with advanced bronchial carcinomas: a novel chance for characterization and treatment monitoring? Eur Radiol 2004;14:1226–33
Tateishi U, Nishihara H, Tsukamoto E, Morikawa T, Tamaki N, Miyasaka K. Lung tumors evaluated with FDG-PET and dynamic CT: the relationship between vascular density and glucose metabolism. J Comput Assist Tomogr 2002;26:185–90
Hoekstra CJ, Stroobants SG, Hoekstra OS, Smit EF, Vansteenkiste JF, Lammertsma AA. Measurement of perfusion in stage IIIA-N2 non-small cell lung cancer using H215O and positron emission tomography. Clin Cancer Res 2002;8:2109–15
Veronesi G, Landoni C, Pelosi G, Picchio M, Sonzogni A, Leon ME, et al. Fluoro-deoxi-glucose uptake and angiogenesis are independent biological features in lung metastases. Br J Cancer 2002;86:1391–5
Mullani N, Herbst R, Abbruzzese J, Charnsangavej C, Kim E, Tran H, et al. Antiangiogenic treatment with endostatin results in uncoupling of blood flow and glucose metabolism in human tumors. Clin Positron Imaging 2000;3:151
Mankoff DA, Dunnwald LK, Gralow JR, Ellis GK, Schubert EK, Tseng J, et al. Changes in blood flow and metabolism in locally advanced breast cancer treated with neoadjuvant chemotherapy. J Nucl Med 2003;44:1806–14
Kurdziel KA, Figg WD, Carrasquillo JA, Huebsch S, Whatley M, Sellers D, et al. Using positron emission tomography 2-deoxy-2-[18F]fluoro-D-glucose, 11CO, and 15O-water for monitoring androgen independent prostate cancer. Mol Imaging Biol 2003;5:86–93
Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004;10:145–7
Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 2002;8 4 Suppl:S62–7
Raghunand N, Gatenby RA, Gillies RJ. Microenvironmental and cellular consequences of altered blood flow in tumours. Br J Radiol 2003;76:11–22
Hicks RJ, Kalff V, MacManus MP, Ware RE, Hogg A, McKenzie AF, et al. 18F-FDG PET provides high-impact and powerful prognostic stratification in staging newly diagnosed non-small cell lung cancer. J Nucl Med 2001;42:1596–604
Benard F, Smith RJ, Hustinx R, Karp JS, Alavi A. Clinical evaluation of processing techniques for attenuation correction with Cs-137 in whole body PET imaging. J Nucl Med 1999;40:1257–63
Erasmus JJ, Gladish GW, Broemeling L, Sabloff BS, Truong MT, Herbst RS, et al. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol 2003;21:2574–82
Miles KA, Griffiths MR, Fuentes MA. Standardized perfusion value: universal CT contrast enhancement scale that correlates with FDG PET in lung nodules. Radiology 2001;220:548–53
Yamashita K, Matsunobe S, Tsuda T, Okuda K, Matsumoto K, Oyanagi H, et al. Intratumoral necrosis of lung carcinoma: a potential diagnostic pitfall in incremental dynamic computed tomography analysis of solitary pulmonary nodules? J Thorac Imaging 1997;12:181–7
Ahuja V, Coleman RE, Herndon J, Patz EF Jr. The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with nonsmall cell lung carcinoma. Cancer 1998;83:918–24
Vansteenkiste JF, Stroobants SG, Dupont PJ, De Leyn PR, Verbeken EK, Deneffe GJ, et al. Prognostic importance of the standardized uptake value on 18F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: an analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol 1999;17:3201–6
Higashi K, Ueda Y, Arisaka Y, Sakuma T, Nambu Y, Oguchi M, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 2002;43:39–45
Jeong HJ, Min JJ, Park JM, Chung JK, Kim BT, Jeong JM, et al. Determination of the prognostic value of [18F] fluorodeoxyglucose uptake by using positron emission tomography in patients with non-small cell lung cancer. Nucl Med Commun 2002;23:865–70
Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol 2004;22:3255–60
Fontanini G, Bigini D, Vignati S, Basolo F, Mussi A, Lucchi M, et al. Microvessel count predicts metastatic disease and survival in non-small cell lung cancer. J Pathol 1995;177:57–63
Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM, et al. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2002;87:694–701
Tanaka F, Yanagihara K, Otake Y, Kawano Y, Miyahara R, Takenaka K, et al. Prognostic factors in resected pathologic (p-) stage IIIA-N2, non-small-cell lung cancer. Ann Surg Oncol 2004;11:612–8
Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 2001;85:881–90
Foo SS, Abbott DF, Lawrentschuk N, Scott AM. Functional imaging of intratumoral hypoxia. Mol Imaging Biol 2004;6:291–305
Rajendran JG, Wilson DC, Conrad EU, Peterson LM, Bruckner JD, Rasey JS, et al. [18F]FMISO and [18F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging 2003;30:695–704
Rajendran JG, Mankoff DA, O’Sullivan F, Peterson LM, Schwartz DL, Conrad EU, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 2004;10:2245–52
Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500–7
Antoch G, Freudenberg LS, Beyer T, Bockisch A, Debatin JF. To enhance or not to enhance? 18F-FDG and CT contrast agents in dual-modality 18F-FDG PET/CT. J Nucl Med 2004;45:56S–65S
Miles KA, Hayball MP, Dixon AK. Colour perfusion imaging: a new application of computed tomography. Lancet 1991;337:643–5
Miles KA, Griffiths MR. Perfusion CT: a worthwhile enhancement? Br J Radiol 2003;76:220–31
Acknowledgements
This study was supported by a grant from the Wesley Research Institute (WRI), Brisbane, Australia. WRI had no involvement in study design; collection, analysis, and interpretation of data; writing of the report, nor in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miles, K.A., Griffiths, M.R. & Keith, C.J. Blood flow–metabolic relationships are dependent on tumour size in non-small cell lung cancer: a study using quantitative contrast-enhanced computer tomography and positron emission tomography. Eur J Nucl Med Mol Imaging 33, 22–28 (2006). https://doi.org/10.1007/s00259-005-1932-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1932-7