Abstract
Purpose
Alzheimer’s disease (AD) is a primary degenerative disease that progressively affects all brain functions, with devastating consequences for the patient, the patient’s family and society. Rest regional cerebral blood flow (rCBF) could have a strategic role in differentiating between AD patients and normal controls, but its use for this purpose has a low discriminatory capacity. The purpose of this study was to evaluate whether the diagnostic sensitivity of rCBF single-photon emission computed tomography (SPECT) could be increased by using an episodic memory task provocation, i.e. memory-provoked rCBF-SPECT (MP-SPECT).
Methods
Eighteen persons (73.2±4.8 years) with mild AD and 18 healthy elderly (69.4±3.9 years) were included in the study. The subjects were injected with 99mTc-hexamethylpropylene amine oxime (HMPAO) during memory provocation with faces and names, followed by an rCBF-SPECT study. The rCBF 99mTc-HMPAO SPECT images were analysed using statistical parametric mapping (SPM2). Peaks with a false discovery rate corrected value of 0.05 were considered significant.
Results
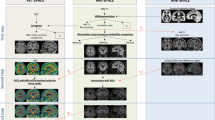
On MP-SPECT, the AD group showed a significant rCBF reduction in the left parietal cortex in comparison with healthy elderly. At rest, no significant group differences were seen.
Conclusion
Memory provocation increased the sensitivity of rCBF-SPECT for the detection of AD-related blood flow changes in the brain at the group level. Further studies are needed to evaluate MP-SPECT as a diagnostic tool at the individual level. If a higher sensitivity for AD at the individual level is verified in future studies, a single MP-SPECT study might be sufficient in the clinical setting.

Similar content being viewed by others
References
Backman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain 2001;124:96–102
Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry 2001;58:853–8
Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology 2000;54:827–32
Citron M. Alzheimer’s disease: treatments in discovery and development. Nat Neurosci Suppl 2002;5:1055–7
Alzheimer A. Über eine eigenartige Erkrangung der Hirnrinde. Allg Z Psychiatr 1907;64:146–8
Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, et al. Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000;54:S4–9
Cummings JL, Benson DF. Dementia: a clinical approach. Boston: Butterworth-Heinemann; 1992
Fratiglioni L, Launer LJ, Andersen K, Breteler MM, Copeland JR, Dartigues JF, et al. Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000;54:S10–5
Waldemar G, Hogh P, Paulson OB. Functional brain imaging with single-photon emission computed tomography in the diagnosis of Alzheimer’s disease. Int Psychogeriatr 1997;9 Suppl 1:223–7; discussion 247–52
Lee BC, Mintun M, Buckner RL, Morris JC. Imaging of Alzheimer’s disease. J Neuroimaging 2003;13:199–214
Benson DF, Kuhl DE, Hawkins RA, Phelps ME, Cummings JL, Tsai SY. The fluorodeoxyglucose18F scan in Alzheimer’s disease and multi-infarct dementia. Arch Neurol 1983;40:711–4
Duara R, Grady C, Haxby J, Sundaram M, Cutler NR, Heston L, et al. Positron emission tomography in Alzheimer’s disease. Neurology 1986;36:879–87
Lee AC, Rahman S, Hodges JR, Sahakian BJ, Graham KS. Associative and recognition memory for novel objects in dementia: implications for diagnosis. Eur J Neurosci 2003;18:1660–70
Hoffmann J, Twiesselmann C, Kummer MP, Romagnoli P, Herzog V. A possible role for the Alzheimer amyloid precursor protein in the regulation of epidermal basal cell proliferation. Eur J Cell Biol 2000;79:905–14
Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl 1996;165:3–12
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004;55:306–19
Wang Y, Klunk WE, Debnath ML, Huang GF, Holt DP, Shao L, et al. Development of a PET/SPECT agent for amyloid imaging in Alzheimer’s disease. J Mol Neurosci 2004;24:55–62
Elgh E, Sundstrom T, Nasman B, Ahlstrom R, Nyberg L. Memory functions and rCBF 99mTc-HMPAO SPET: developing diagnostics in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2002;29:1140–8
Rombouts SA, Barkhof F, Veltman DJ, Machielsen WC, Witter MP, Bierlaagh MA, et al. Functional MR imaging in Alzheimer’s disease during memory encoding. Am J Neuroradiol 2000;21:1869–75
Nobili F, Brugnolo A, Calvini P, Copello F, De Leo C, Girtler N, et al. Resting SPECT–neuropsychology correlation in very mild Alzheimer’s disease. Clin Neurophysiol 2005;116:364–75
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–72
Meikle SR, Hutton BF, Bailey DL. A transmission-dependent method for scatter correction in SPECT. J Nucl Med 1994;35:360–7
Van Laere KJ, Versijpt J, Koole M, Vandenberghe S, Lahorte P, Lemahieu I, et al. Experimental performance assessment of SPM for SPECT neuroactivation studies using a subresolution sandwich phantom design. NeuroImage 2002;16:200–16
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imag 1994;13:601–9
Tatsch K, Asenbaum S, Bartenstein P, Catafau A, Halldin C, Pilowsky LS, et al. European Association of Nuclear Medicine procedure guidelines for brain perfusion SPET using 99mTc-labelled radiopharmaceuticals. Eur J Nucl Med Mol Imaging 2002;29:BP36–42
Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 2002;15:870–8
Lassen NA, Andersen AR, Friberg L, Paulson OB. The retention of [99mTc]-d,l-HM-PAO in the human brain after intracarotid bolus injection: a kinetic analysis. J Cereb Blood Flow Metab 1988;8:S13–22
Buschke H. Cued recall in amnesia. J Clin Neuropsychol 1984;6:433–40
Meyers JE, Meyers KR. Rey Complex Figure Test under four different administration procedures. Clin Neuropsychol 1995:63–67 JN: Clinical-Neu
Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol 1999;12:168–79
Nilsson L-G, Bäckman L, Erngrund K, Nyberg L, Adolfsson R, Bucht G, et al. The Betula prospective cohort study: memory, health, and aging. Aging Neuropsychol Cogn 1997:1–32
Spreen O, Strauss E. A compendium of neuropsychological tests. New York: Oxford University Press; 1991
Wechsler D. Wechsler Adult Intelligence Scale-Revised (WAIS-R). New York:: The Psychological Corporation; 1981
Kaplan E, Fein D, Morris R, Delis D. WAIS-R as a neuropsychological instrument. New York: The Psychological Corporation; 1991
Reitan RM. Trail Making Test: manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1992
Nordberg A. Toward an early diagnosis and treatment of Alzheimer’s disease. Int Psychogeriatr 2003;15:223–37
Encinas M, De Juan R, Marcos A, Gil P, Barabash A, Fernandez C, et al. Regional cerebral blood flow assessed with 99mTc-ECD SPET as a marker of progression of mild cognitive impairment to Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2003;30:1473–80
Kaneko K, Kuwabara Y, Sasaki M, Ogomori K, Ichimiya A, Koga H, et al. Posterior cingulate hypoperfusion in Alzheimer’s disease, senile dementia of Alzheimer type, and other dementias evaluated by three-dimensional stereotactic surface projections using Tc-99m HMPAO SPECT. Clin Nucl Med 2004;29:362–6
Elgh E, Larsson A, Eriksson S, Nyberg L. Altered prefrontal brain activity in persons at risk for Alzheimer’s disease: an fMRI study. Int Psychogeriatr 2003;15:121–33
Sayit E, Yener G, Capa G, Ertay T, Keskin B, Fadiloglu S, et al. Basal and activational 99Tcm-HMPAO brain SPECT in Alzheimer’s disease. Nucl Med Commun 2000;21:763–8
Beversdorf D, Metzger S, Nelson D, Alonso R, Kight J. Single-word auditory stimulation and regional cerebral blood flow as studied by SPECT. Psychiatry Res 1995;61:181–9
Cardebat D, Demonet JF, Puel M, Agniel A, Viallard G, Celsis P. Brain correlates of memory processes in patients with dementia of Alzheimer’s type: a SPECT activation study. J Cereb Blood Flow Metab 1998;18:457–62
Tonini G, Shanks MF, Venneri A. Short-term longitudinal evaluation of cerebral blood flow in mild Alzheimer’s disease. Neurol Sci 2003;24:24–30
Garrido GE, Furuie SS, Buchpiguel CA, Bottino CM, Almeida OP, Cid CG, et al. Relation between medial temporal atrophy and functional brain activity during memory processing in Alzheimer’s disease: a combined MRI and SPECT study. J Neurol Neurosurg Psychiatry 2002;73:508–16
Zheng XM. Detecting regional cerebral blood flow changes in Alzheimer’s patients after milameline treatment: activation or baseline SPECT? J Nucl Med Technol 2002;30:118–22
Backman L, Andersson JL, Nyberg L, Winblad B, Nordberg A, Almkvist O. Brain regions associated with episodic retrieval in normal aging and Alzheimer’s disease. Neurology 1999;52:1861–70
Ryding E. SPECT measurements of brain function in dementia; a review. Acta Neurol Scand Suppl 1996;168:54–8
Jagust WJ. Functional imaging patterns in Alzheimer’s disease. Relationships to neurobiology. Ann NY Acad Sci 1996;777:30–6
McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 2001;58:1803–9
Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc 2001;7:631–9
Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology 2000;55:1847–53
Grady CL, Haxby JV, Horwitz B, Sundaram M, Berg G, Schapiro M, et al. Longitudinal study of the early neuropsychological and cerebral metabolic changes in dementia of the Alzheimer type. J Clin Exp Neuropsychol 1988;10:576–96
Lafleche G, Albert M. Executive function deficits in mild Alzheimer’s disease. Neuropsychology 1995;9:313–20
Kato T, Knopman D, Liu H. Dissociation of regional activation in mild AD during visual encoding: a functional MRI study. Neurology 2001;57:812–6
Gilleard CJ. Education and Alzheimer’s disease: a review of recent international epidemiological studies. Aging Ment Health 1997;1:33–6
De Jager CA, Hogervorst E, Combrinck M, Budge MM. Sensitivity and specificity of neuropsychological tests for mild cognitive impairment, vascular cognitive impairment and Alzheimer’s disease. Psychol Med 2003;33:1039–50
Lovden M, Ronnlund M, Wahlin A, Backman L, Nyberg L, Nilsson LG. The extent of stability and change in episodic and semantic memory in old age: demographic predictors of level and change. J Gerontol B Psychol Sci Soc Sci 2004;59:P130–4
Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol 1992;32:371–5
Acknowledgements
Financial support was provided by Lion, KK (kunskap och kompetens), the Medical Faculty, Umeå University, the Swedish Research Council and the Borgerskapet of Umeå Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sundström, T., Elgh, E., Larsson, A. et al. Memory-provoked rCBF-SPECT as a diagnostic tool in Alzheimer’s disease?. Eur J Nucl Med Mol Imaging 33, 73–80 (2006). https://doi.org/10.1007/s00259-005-1874-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1874-0