Abstract
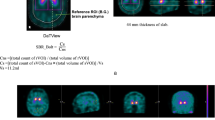
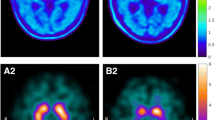
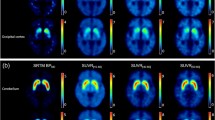
A comparative study was carried out on two promising presynaptic dopamine transporter single-photon emission tomography (SPECT) radioligands with a fast pharmacokinetic profile, 123I-FP-β-CIT (FP) and 99mTc-TRODAT-1 (TR), in order to assess their differential diagnostic power in early parkinsonism and their sensitivity for detection of disease progression. This cross-sectional study was conducted on 96 patients with early-stage parkinsonism referred in a tertiary clinical setting. Mean disease duration was 2.0±1.3 years, and patients had a modified Hoehn and Yahr (H&Y) stage of 1–2 (average 1.2). Forty-seven patients received TR, and 49 received FP. In both groups, ten patients with normal presynaptic function were included as a control population; all other patients were clinically diagnosed as having idiopathic Parkinson’s disease. Groups were matched for gender, age, disease duration and modified H&Y stage. Triple-head gamma camera SPECT was analysed using a semiquantitative index of transporter binding (BI). Discriminant analysis with cross-validation resulted in a maximal classification accuracy for FP of 93% (sensitivity 95% and specificity 86%) for the contralateral putamen BI. For TR, the corresponding values were 87% accuracy, 92% sensitivity and 70% specificity. For FP, disease duration was correlated with both the putamen BI (−8.8%/year, ρ=−0.41, P=0.025) and the putamen/caudate ratio (−7.4%/year, ρ=−0.51, P=0.004), but for TR no significant correlation was found (all P values >0.5). In conclusion, both FP and TR show high sensitivity in a clinically relevant setting, but FP has superior accuracy for early differential diagnosis of idiopathic parkinsonism and non-degenerative extrapyramidal disorders, as well as better sensitivity for disease follow-up.



Similar content being viewed by others
References
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55:181–184.
Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999; 56:33–39.
Brooks DJ. Imaging end points for monitoring neuroprotection in Parkinson’s disease. Ann Neurol 2003; 53 Suppl 3:S110–S118.
Parkinson Study Group. A multicenter assessment of dopamine transporter imaging with DOPASCAN/SPECT in parkinsonism. Neurology 2000; 55:1540–1547.
Booij J, Tissingh G, Winogrodzka A, van Royen EA. Imaging of the dopaminergic neurotransmission system using single-photon emission tomography and positron emission tomography in patients with parkinsonism. Eur J Nucl Med 1999; 26:171–182.
Kung HF, Kung MP, Choi SR. Radiopharmaceuticals for single-photon emission computed tomography brain imaging. Semin Nucl Med 2003; 33:2–13.
Benamer TS, Patterson J, Grosset DG, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT Study Group. Mov Disord 2000; 15:503–510.
Pirker W, Asenbaum S, Bencsits G, et al. [123I]beta-CIT SPECT in multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Mov Disord 2000; 15:1158–1167.
Brucke T, Kornhuber J, Angelberger P, Asenbaum S, Frassine H, Podreka I. SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT. Binding kinetics in the human brain. J Neural Transm Gen Sect 1993; 94:137–146.
Tissingh G, Bergmans P, Winogrodzka A, et al. Nigrostriatal dopaminergic imaging with iodine-123-beta CIT-FP/SPECT and fluorine-18-FDOPA/PET. J Nucl Med 1997; 38:1271–1272.
Fischman AJ, Bonab AA, Babich JW, et al. Rapid detection of Parkinson’s disease by SPECT with altropane: a selective ligand for dopamine transporters. Synapse 1998; 29:128–141.
Seibyl JP, Marek K, Sheff K, et al. Iodine-123-beta-CIT and iodine-123-FPCIT SPECT measurement of dopamine transporters in healthy subjects and Parkinson’s patients. J Nucl Med 1998; 39:1500–1508.
Kung HF. Development of Tc-99m labeled tropanes: TRODAT-1, as a dopamine transporter imaging agent. Nucl Med Biol 2001; 28:505–508.
Huang WS, Lin SZ, Lin JC, Wey SP, Ting G, Liu RS. Evaluation of early-stage Parkinson’s disease with99mTc-TRODAT-1 imaging. J Nucl Med 2001; 42:1303–1308.
Johannsen B, Pietzsch HJ. Development of technetium-99m-based CNS receptor ligands: have there been any advances? Eur J Nucl Med Mol Imaging 2002; 29:263–275.
Maes A, Vanbilloen H, Cleynhens B, Mortelmans L, Bormans G, Verbruggen A.99mTc-M-TRODAT is not superior to 99mTc-TRODAT-1 for the diagnosis of Parkinson’s disease. Eur J Nucl Med Mol Imaging 2003; 30:796–797.
Huang WS, Chiang YH, Lin JC, Chou YH, Cheng CY, Liu RS. Crossover Study of99mTc-TRODAT-1 SPECT and 18F-FDOPA PET in Parkinson’s disease patients. J Nucl Med 2003; 44:999–1005.
Hoehn M, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967; 17:427–442.
Meegalla SK, Plossl K, Kung MP, et al. Synthesis and characterization of technetium-99m-labeled tropanes as dopamine transporter-imaging agents. J Med Chem 1997; 40:9–17.
Cleynhens J, Vanbilloen H, Bormans G, de Groot T, Verbruggen A. Synthesis and isolation of the diastereomers of 4-tolyl,4-ethylphenyl- and 2,4-dimethylphenyl derivatives of99mTc-TRODAT-1. J Labelled Compd Radiopharm 2001; 44:S529–S531.
Kushner SA, McElgin WT, Kung MP, et al. Kinetic modeling of [99mTc]TRODAT-1: a dopamine transporter imaging agent. J Nucl Med 1999; 40:150–158.
Booij J, Hemelaar TG, Speelman JD, de Bruin K, Janssen AG, van Royen EA. One-day protocol for imaging of the nigrostriatal dopaminergic pathway in Parkinson’s disease by [123I]FPCIT SPECT. J Nucl Med 1999; 40:753–761.
Kao PF, Tzen KY, Yen TC, et al. The optimal imaging time for [99Tcm]TRODAT-1/SPET in normal subjects and patients with Parkinson’s disease. Nucl Med Commun 2001; 22:151–154.
Booij J, Speelman JD, Horstink MW, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med 2001; 28:266–272.
Efron B. Estimating the error rate of a prediction rule: improvements on cross-validation. J Am Stat Assoc 1983; 78:316–331.
Mozley PD, Schneider JS, Acton PD, et al. Binding of [99mTc]TRODAT-1 to dopamine transporters in patients with Parkinson’s disease and in healthy volunteers. J Nucl Med 2000; 41:584–589.
Burn DJ, Sawle GV, Brooks DJ. Differential diagnosis of Parkinson’s disease, multiple system atrophy, and Steele-Richardson-Olszewski syndrome: discriminant analysis of striatal18F-dopa PET data. J Neurol Neurosurg Psychiatry 1994; 57:278–284.
Meyer PT, Sattler B, Lincke T, Seese A, Sabri O. Investigating dopaminergic neurotransmission with123I-FP-CIT SPECT: comparability of modern SPECT systems. J Nucl Med 2003; 44:839–845.
Van Laere K, Koole M, Versijpt J, et al. Transfer of normal99mTc-ECD brain SPET databases between different gamma cameras. Eur J Nucl Med 2001; 28:435–449.
Jankovic J, Rajput AH, McDermott MP, Perl DP. The evolution of diagnosis in early Parkinson disease. Parkinson Study Group. Arch Neurol 2000; 57:369–372.
Winogrodzka A, Bergmans P, Booij J, van Royen EA, Janssen AG, Wolters EC. [123I]FP-CIT SPECT is a useful method to monitor the rate of dopaminergic degeneration in early-stage Parkinson’s disease. J Neural Transm 2001; 108:1011–1019.
Marek K, Innis R, van Dyck C, et al. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology 2001; 57:2089–2094.
Pirker W, Djamshidian S, Asenbaum S, et al. Progression of dopaminergic degeneration in Parkinson’s disease and atypical parkinsonism: a longitudinal beta-CIT SPECT study. Mov Disord 2002; 17:45–53.
Winogrodzka A, Bergmans P, Booij J, van Royen EA, Stoof JC, Wolters EC. [123I]beta-CIT SPECT is a useful method for monitoring dopaminergic degeneration in early stage Parkinson’s disease. J Neurol Neurosurg Psychiatry 2003; 74:294–298.
Parkinson Study Group. A randomized controlled trial comparing pramipexole with levodopa in early Parkinson’s disease: design and methods of the CALM-PD Study. Clin Neuropharmacol 2000; 23:34–44.
Hardie RJ. Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F] dopa PET. J Neurol Neurosurg Psychiatry 1999; 66:256.
Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson’s disease. Brain 1996; 119 (Pt 2):585–591.
Nurmi E, Ruottinen HM, Bergman J, et al. Rate of progression in Parkinson’s disease: a 6-[18F]fluoro-L-dopa PET study. Mov Disord 2001; 16:608–615.
Reader TA, Ase AR, Huang N, Hebert C, van Gelder NM. Neuroleptics and dopamine transporters. Neurochem Res 1998; 23:73–80.
Grunder G, Vernaleken I, Muller MJ, et al. Subchronic haloperidol downregulates dopamine synthesis capacity in the brain of schizophrenic patients in vivo. Neuropsychopharmacology 2003; 28:787–794.
Innis RB, Marek KL, Sheff K, et al. Effect of treatment withl-dopa/carbidopa or l-selegiline on striatal dopamine transporter SPECT imaging with [123I]beta-CIT. Mov Disord 1999; 14:436–442.
Van Laere K, Warwick J, Versijpt J, et al. Analysis of clinical SPECT data based on anatomical standardization and reference to normal data: a ROC-based comparison of visual, semiquantitative and voxel-based methods. J Nucl Med 2002; 43:458–469.
Acknowledgements
This study was supported by a research grant from the Leuven University Research Foundation (BOF). The authors thank Prof. H. Kung (Penn University, Philadelphia) for his kind co-operation in the synthesis of 99mTc-TRODAT-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Laere, K., De Ceuninck, L., Dom, R. et al. Dopamine transporter SPECT using fast kinetic ligands: 123I-FP-β-CIT versus 99mTc-TRODAT-1. Eur J Nucl Med Mol Imaging 31, 1119–1127 (2004). https://doi.org/10.1007/s00259-004-1480-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1480-6