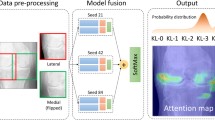
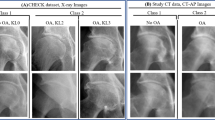
Abstract
Purpose
To develop a deep learning model to distinguish rheumatoid arthritis (RA) from osteoarthritis (OA) using hand radiographs and to evaluate the effects of changing pretraining and training parameters on model performance.
Materials and methods
A convolutional neural network was retrospectively trained on 9714 hand radiograph exams from 8387 patients obtained from 2017 to 2021 at seven hospitals within an integrated healthcare network. Performance was assessed using an independent test set of 250 exams from 146 patients. Binary discriminatory capacity (no arthritis versus arthritis; RA versus not RA) and three-way classification (no arthritis versus OA versus RA) were evaluated. The effects of additional pretraining using musculoskeletal radiographs, using all views as opposed to only the posteroanterior view, and varying image resolution on model performance were also investigated. Area under the receiver operating characteristic curve (AUC) and Cohen’s kappa coefficient were used to evaluate diagnostic performance.
Results
For no arthritis versus arthritis, the model achieved an AUC of 0.975 (95% CI: 0.957, 0.989). For RA versus not RA, the model achieved an AUC of 0.955 (95% CI: 0.919, 0.983). For three-way classification, the model achieved a kappa of 0.806 (95% CI: 0.742, 0.866) and accuracy of 87.2% (95% CI: 83.2%, 91.2%) on the test set. Increasing image resolution increased performance up to 1024 × 1024 pixels. Additional pretraining on musculoskeletal radiographs and using all views did not significantly affect performance.
Conclusion
A deep learning model can be used to distinguish no arthritis, OA, and RA on hand radiographs with high performance.




Similar content being viewed by others
References
Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68(1):1–25. https://doi.org/10.1002/acr.22783.
Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99. https://doi.org/10.1136/annrheumdis-2019-216655.
Lard LR, Visser H, Speyer I, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001;111(6):446–51. https://doi.org/10.1016/s0002-9343(01)00872-5.
Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int. 2017;37(9):1551–7. https://doi.org/10.1007/s00296-017-3726-1.
Crowson CS, Matteson EL, Myasoedova E, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63(3):633–9. https://doi.org/10.1002/art.30155.
Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26(1):77–90. https://doi.org/10.1185/03007990903422307.
Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthr Cartil. 2022;30(1):10–6. https://doi.org/10.1016/j.joca.2021.05.007.
Safiri S, Kolahi A-A, Smith E, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819–28. https://doi.org/10.1136/annrheumdis-2019-216515.
Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. https://doi.org/10.1002/art.23176.
Litwic A, Edwards M, Dennison E, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99. https://doi.org/10.1093/bmb/lds038.
Haugen IK, Englund M, Aliabadi P, et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70(9):1581–6. https://doi.org/10.1136/ard.2011.150078.
Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthr Cartil. 2022;30(2):184–95. https://doi.org/10.1016/j.joca.2021.04.020.
Larson DB, Chen MC, Lungren MP, Halabi SS, Stence NV, Langlotz CP. Performance of a Deep-learning neural network model in assessing skeletal maturity on pediatric hand radiographs. Radiology. 2018;287(1):313–22. https://doi.org/10.1148/radiol.2017170236.
He Y, Pan I, Bao B, et al. Deep learning-based classification of primary bone tumors on radiographs: a preliminary study. eBioMedicine. Elsevier. 2020:62. https://doi.org/10.1016/j.ebiom.2020.103121.
Carmo LO, van den Merkhof A, Olczak J, et al. An increasing number of convolutional neural networks for fracture recognition and classification in orthopaedics : are these externally validated and ready for clinical application? Bone Jt Open. 2021;2(10):879–85. https://doi.org/10.1302/2633-1462.210.BJO-2021-0133.
Olsson S, Akbarian E, Lind A, Razavian AS, Gordon M. Automating classification of osteoarthritis according to Kellgren-Lawrence in the knee using deep learning in an unfiltered adult population. BMC Musculoskelet Disord. 2021;22(1):844. https://doi.org/10.1186/s12891-021-04722-7.
Murakami S, Hatano K, Tan J, Kim H, Aoki T. Automatic identification of bone erosions in rheumatoid arthritis from hand radiographs based on deep convolutional neural network. Multimed Tools Appl. 2018;77(9):10921–37. https://doi.org/10.1007/s11042-017-5449-4.
Betancourt-Hernández M, Viera-López G, Serrano-Muñoz A. Automatic diagnosis of rheumatoid arthritis from hand radiographs using convolutional neural networks. Revista Cubana de Física, [S.l.]. 2018;35(1):39–43.
Üreten K, Erbay H, Maraş HH. Detection of rheumatoid arthritis from hand radiographs using a convolutional neural network. Clin Rheumatol. 2020;39(4):969–74. https://doi.org/10.1007/s10067-019-04487-4.
Üreten K, Maraş HH. Automated classification of rheumatoid arthritis, osteoarthritis, and normal hand radiographs with deep learning methods. J Digit Imaging. 2022;35(2):193–9. https://doi.org/10.1007/s10278-021-00564-w.
Tan M, Le QV. EfficientNet: rethinking model scaling for convolutional neural networks. arXiv; 2020. http://arxiv.org/abs/1905.11946. Accessed May 14, 2022.
Deng J, Dong W, Socher R, Li LJ, Li K, Fei-Fei L. ImageNet: a large-scale hierarchical image database. In: 2009 IEEE Conference on Computer Vision and Pattern Recognition. 2009. p. 248–55. https://doi.org/10.1109/CVPR.2009.5206848.
Rajpurkar P, Irvin J, Bagul A, et al. MURA: large dataset for abnormality detection in musculoskeletal radiographs. arXiv; 2018. http://arxiv.org/abs/1712.06957. Accessed May 14, 2022.
Sabottke CF, Spieler BM. The effect of image resolution on deep learning in radiography. Radiology: Artificial Intelligence. Radiological Society of North America; 2020;2(1):e190015. https://doi.org/10.1148/ryai.2019190015.
Hirano T, Nishide M, Nonaka N, et al. Development and validation of a deep-learning model for scoring of radiographic finger joint destruction in rheumatoid arthritis. Rheumatol Adv Pract. 2019;3(2):rkz047. https://doi.org/10.1093/rap/rkz047.
Fuchs HA, Kaye JJ, Callahan LF, Nance EP, Pincus T. Evidence of significant radiographic damage in rheumatoid arthritis within the first 2 years of disease. J Rheumatol. 1989;16(5):585–91.
van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35(1):26–34. https://doi.org/10.1002/art.1780350105.
England BR. Clinical manifestations of rheumatoid arthritis. UpToDate. Waltham, MA; https://www.uptodate.com/contents/clinical-manifestations-of-rheumatoid-arthritis. Accessed June 26; 2022.
Baker JF. Diagnosis and differential diagnosis of rheumatoid arthritis. UpToDate. Waltham, MA; https://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-rheumatoid-arthritis. Accessed June 26; 2022.
Bohndorf K, Schalm J. Diagnostic radiography in rheumatoid arthritis: benefits and limitations. Baillieres Clin Rheumatol. 1996;10(3):399–407. https://doi.org/10.1016/s0950-3579(96)80038-0.
Coffey CM, Crowson CS, Myasoedova E, Matteson EL, Davis JM. Evidence of diagnostic and treatment delay in seronegative rheumatoid arthritis: missing the window of opportunity. Mayo Clin Proc. 2019;94(11):2241–8. https://doi.org/10.1016/j.mayocp.2019.05.023.
Boeters DM, Gaujoux-Viala C, Constantin A, van der Helm-van Mil AHM. The 2010 ACR/EULAR criteria are not sufficiently accurate in the early identification of autoantibody-negative rheumatoid arthritis: results from the Leiden-EAC and ESPOIR cohorts. Semin Arthritis Rheum. 2017;47(2):170–4. https://doi.org/10.1016/j.semarthrit.2017.04.009.
Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology. 2012;51(suppl_6):vi5–9. https://doi.org/10.1093/rheumatology/kes279s.
McQueen FM. The use of MRI in early RA. Rheumatology (Oxford). 2008;47(11):1597–9. https://doi.org/10.1093/rheumatology/ken332.
Terslev L, Torp-Pedersen S, Savnik A, et al. Doppler ultrasound and magnetic resonance imaging of synovial inflammation of the hand in rheumatoid arthritis: a comparative study. Arthritis Rheum. 2003;48(9):2434–41. https://doi.org/10.1002/art.11245.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Pan, I., Kim, S.Y. et al. Deep learning discrimination of rheumatoid arthritis from osteoarthritis on hand radiography. Skeletal Radiol 53, 377–383 (2024). https://doi.org/10.1007/s00256-023-04408-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-023-04408-2