Abstract
Objective
To assess the diagnostic contribution of contrast-enhanced 3D STIR (ce3D-SS) high-resolution magnetic resonance (MR) imaging of peripheral nerve pathology relative to conventional 2D sequences.
Materials and methods
In this IRB-approved retrospective study, two radiologists reviewed 60 MR neurography studies with nerve pathology findings. The diagnostic contribution of ce3D-SS imaging was scored on a 4-point Likert scale (1 = no additional information, 2 = supports interpretation, 3 = moderate additional information, and 4 = diagnosis not possible without ce3D-SS). Image quality, nerve visualization, and detection of nerve pathology were also assessed for both standard 2D neurography and ce3D-SS sequences utilizing a 3-point Likert scale. Descriptive statistics are reported.
Results
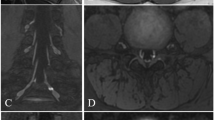
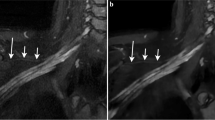
The diagnostic contribution score for ce3D-SS imaging was 2.25 for the brachial plexus, 1.50 for extremities, and 1.75 for the lumbosacral plexus. For brachial plexus, the mean consensus scores for image quality, nerve visualization, and detection of nerve pathology were 2.55, 2.5, and 2.55 for 2D and 2.35, 2.45, and 2.45 for 3D. For extremities, the mean consensus scores for image quality, nerve visualization, and detection of nerve pathology were 2.60, 2.80, and 2.70 for 2D and 1.8, 2.20, and 2.10 for 3D. For lumbosacral plexus, the mean consensus scores for image quality, nerve visualization, and detection of nerve pathology were 2.45, 2.75, and 2.65 for 2D and 2.0, 2.45, and 2.25 for 3D.
Conclusion
Overall, our study supports the potential application of ce3D-SS imaging for MRN of the brachial plexus but suggests that 2D MRN protocols are sufficient for MRN of the extremities and lumbosacral plexus.





Similar content being viewed by others
References
Sneag DB, Queler S. Technological advancements in magnetic resonance neurography. Curr Neurol Neurosci Rep. 2019;19(10):75. https://doi.org/10.1007/s11910-019-0996-x.
Chhabra A, Thawait GK, Soldatos T, Thakkar RS, Del Grande F, Chalian M, Carrino JA. High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradiol. 2013;34(3):486–97. https://doi.org/10.3174/ajnr.A3287.
Chhabra A, Rozen S, Scott K. Three-dimensional MR neurography of the lumbosacral plexus. Semin Musculoskelet Radiol. 2015;19(2):149–59. https://doi.org/10.1055/s-0035-1545077.
Mazal AT, Faramarzalian A, Samet JD, Gill K, Cheng J, Chhabra A. MR neurography of the brachial plexus in adult and pediatric age groups: evolution, recent advances, and future directions. Expert Rev Med Devices. 2020;17(2):111–22. https://doi.org/10.1080/17434440.2020.1719830.
Chhabra A. Peripheral MR neurography: approach to interpretation. Neuroimaging Clin N Am. 2014;24(1):79–89. https://doi.org/10.1016/j.nic.2013.03.033.
Harrell AD, Johnson D, Samet J, Omar IM, Deshmukh S. With or without? A retrospective analysis of intravenous contrast utility in magnetic resonance neurography. Skeletal Radiol. 2020;49(4):577–84. https://doi.org/10.1007/s00256-019-03321-x.
Wang L, Niu Y, Kong X, Yu Q, Kong X, Lv Y, Shi H, Li C, Wu W, Wang B, Liu D. The application of paramagnetic contrast-based T2 effect to 3D heavily T2W high-resolution MR imaging of the brachial plexus and its branches. Eur J Radiol. 2016;85(3):578–84. https://doi.org/10.1016/j.ejrad.2015.12.001.
Chen WC, Tsai YH, Weng HH, Wang SC, Liu HL, Peng SL, Chen CF. Value of enhancement technique in 3D–T2-STIR images of the brachial plexus. J Comput Assist Tomogr. 2014;38(3):335–9. https://doi.org/10.1097/RCT.0000000000000061.
Sneag DB, Daniels SP, Geannette C, Queler SC, Lin BQ, de Silva C, Tan ET. Post-contrast 3D inversion recovery magnetic resonance neurography for evaluation of branch nerves of the brachial plexus. Eur J Radiol. 2020;132:109304. https://doi.org/10.1016/j.ejrad.2020.109304.
Viallon M, Vargas MI, Jlassi H, Lövblad KO, Delavelle J. High-resolution and functional magnetic resonance imaging of the brachial plexus using an isotropic 3D T2 STIR (Short Term Inversion Recovery) SPACE sequence and diffusion tensor imaging. Eur Radiol. 2008;18(5):1018–23. https://doi.org/10.1007/s00330-007-0834-4.
Xu Z, Zhang T, Chen J, Liu Z, Wang T, Hu Y, Zhang J, Xue F. Combine contrast-enhanced 3D T2-weighted short inversion time inversion recovery MR neurography with MR angiography at 1.5 T in the assessment of brachial plexopathy. MAGMA. 2020. https://doi.org/10.1007/s10334-020-00867-z.
Zhang Y, Kong X, Zhao Q, Liu X, Gu Y, Xu L. Enhanced MR neurography of the lumbosacral plexus with robust vascular suppression and improved delineation of its small branches. Eur J Radiol. 2020;129:109128. https://doi.org/10.1016/j.ejrad.2020.109128.
Zhang X, Li M, Guan J, Wang H, Li S, Guo Y, Liu M. Evaluation of the sacral nerve plexus in pelvic endometriosis by three-dimensional MR neurography. J Magn Reson Imaging. 2017;45(4):1225–31. https://doi.org/10.1002/jmri.25435.
Chhabra A, Soldatos T, Subhawong TK, Machado AJ, Thawait SK, Wang KC, Padua A Jr, Flammang AJ, Williams EH, Carrino JA. The application of three-dimensional diffusion-weighted PSIF technique in peripheral nerve imaging of the distal extremities. J Magn Reson Imaging. 2011;34(4):962–7. https://doi.org/10.1002/jmri.22684.
Zare M, Faeghi F, Hosseini A, Ardekani MS, Heidari MH, Zarei E. Comparison between three-dimensional diffusion-weighted PSIF technique and routine imaging sequences in evaluation of peripheral nerves in healthy people. Basic Clin Neurosci. 2018;9(1):65–71. https://doi.org/10.29252/NIRP.BCN.9.1.65.
De Paepe KN, Higgins DM, Ball I, Morgan VA, Barton DP, deSouza NM. Visualizing the autonomic and somatic innervation of the female pelvis with 3D MR neurography: a feasibility study. Acta Radiol. 2020;61(12):1668–76. https://doi.org/10.1177/0284185120909337.
Zhai H, Lv Y, Kong X, Liu X, Liu D. Magnetic resonance neurography appearance and diagnostic evaluation of peripheral nerve sheath tumors. Sci Rep. 2019;9(1):6939. https://doi.org/10.1038/s41598-019-43450-w.
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deshmukh, S., Tegtmeyer, K., Kovour, M. et al. Diagnostic contribution of contrast-enhanced 3D MR imaging of peripheral nerve pathology. Skeletal Radiol 50, 2509–2518 (2021). https://doi.org/10.1007/s00256-021-03816-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03816-6