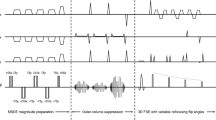
Abstract
Objective
To evaluate ferumoxytol-enhanced vascular suppression for visualizing branch nerves of the brachial plexus in magnetic resonance (MR) neurography.
Materials and methods
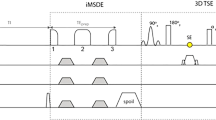
Signal simulations were performed to determine ferumoxytol’s effect on nerve-, fat-, and blood-to-muscle contrast and to optimize pulse sequence parameters. Prospective, in vivo assessment included 10 subjects with chronic anemia who underwent a total of 19 (9 bilateral) pre- and post-infusion brachial plexus exams using three-dimensional (3D), T2-weighted short-tau inversion recovery (T2-STIR) sequences at 3.0 T. Two musculoskeletal radiologists qualitatively rated sequences for the degree of vascular suppression and brachial plexus branch nerve conspicuity. Nerve-to-muscle, -fat, and -vessel contrast ratios were measured.
Results
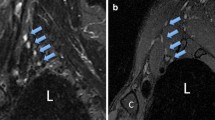
Quantitative nerve/muscle and nerve/small vessel contrast ratios (CRs) increased with ferumoxytol (p < 0.05). Qualitative vascular suppression and suprascapular nerve visualization improved following ferumoxytol administration for both raters (p < .05). Pre- and post-ferumoxytol exams demonstrated moderate to near-perfect inter-rater agreement for nerve visualization and diagnostic confidence for the suprascapular and axillary nerves but poor to no agreement for the long thoracic nerve.
Conclusion
Ferumoxytol in T2-weighted brachial plexus MR neurography provides robust vascular suppression and aids visualization of the suprascapular nerve in volunteers without neuropathy.







Similar content being viewed by others
Abbreviations
- CR:
-
Contrast ratio
- IDA:
-
Iron deficiency anemia
- TE:
-
Echo time
- TI:
-
Inversion time
- TR:
-
Repetition time
References
Filler A. Magnetic resonance neurography and diffusion tensor imaging: origins, history, and clinical impact of the first 50,000 cases with an assessment of efficacy and utility in a prospective 5000-patient study group. Neurosurgery. 2009;65(4 Suppl):A29-43.
Does MD, Snyder RE. T2 relaxation of peripheral nerve measured in vivo. Magn Reson Imaging. 1995;13(4):575–80.
Yoneyama M, Takahara T, Kwee TC, et al. Rapid high resolution MR neurography with a diffusion-weighted pre-pulse. Magn Reson Med Sci. 2013;12:111–9.
Cervantes B, Kirschke JS, Klupp E, et al. Orthogonally combined motion- and diffusion-sensitized driven equilibrium (OC-MDSDE) preparation for vessel signal suppression in 3D turbo spin echo imaging of peripheral nerves in the extremities. Magn Reson Med. 2018;79:407–15.
Chhabra A, Subhawong TK, Bizzell C, et al. 3T MR neurography using three dimensional diffusion-weighted PSIF: technical issues and advantages. Skeletal Radiol. 2011;40:1355–60.
Xu X et al. Investigation of 3D reduced field of view carotid atherosclerotic plaque imaging. Magn Reson Imaging. 2017.
Lindenholz A, et al. The use and pitfalls of intracranial vessel wall imaging: how we do it. Radiology. 2017;286(1):12–28.
Abdel-Aty H, Simonetti O, Friedrich MG. T2-weighted cardiovascular magnetic resonance imaging. J Magn Reson Imaging. 2007;26(3):452–9.
Brown R, et al. Effect of blood flow on double inversion recovery vessel wall MRI of the peripheral arteries: quantitation with T2 mapping and comparison with flow-insensitive T2-prepared inversion recovery imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2010;63(3):736–44.
Deshmukh S, Fayad LM, Ahlawat S. MR neurography (MRN) of the long thoracic nerve: retrospective review of clinical findings and imaging results at our institution over 4 years. Skeletal Radiol. 2017;46(11):1531–40.
Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259–67.
Chen WC, Tsai YH, Weng HH, et al. Value of enhancement technique in 3D–T2 STIR images of the brachial plexus. J Comput Assist Tomogr. 2014;38:335–9.
Wang L, Niu Y, Kong X, et al. The application of paramagnetic contrast-based T2 effect to 3D heavily T2W high-resolution MR imaging of the brachial plexus and its branches. Eur J Radiol. 2016;85:578–84.
Sneag DB, Daniels SP, Geannette C, et al. Post-contrast 3D inversion recovery magnetic resonance neurography for evaluation of branch nerves of the brachial plexus. Eur J Radiol. 2020;28(132):109304.
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834–41.
Ahmad F, Treanor L, McGrath TA, Walker D, McInnes MDF, Schieda N. Safety of off-label use of ferumoxtyol as a contrast agent for MRI: a systematic review and meta-analysis of adverse events. J Magn Reson Imaging. 2021;53(3):840–58.
Alkhunizi SM, Fakhoury M, Abou-Kheir W, Lawand N. Gadolinium retention in the central and peripheral nervous system: implications for pain, cognition, and neurogenesis. Radiology. 2020;297(2):407–16.
Grobner T. Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104–8.
McDonald JS, McDonald RJ. MR imaging safety considerations of gadolinium-based contrast agents: gadolinium retention and nephrogenic systemic fibrosis. Magn Reson Imaging Clin N Am. 2020;28(4):497–507.
Toth GB, Varallyay CG, Horvath A, et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017;92(1):47–66.
Daldrup-Link HE. Ten things you might not know about iron oxide nanoparticles. Radiology. 2017;284(3):616–29.
Knobloch G, Colgan T, Wiens C, et al. Relaxivity of ferumoxytol at 1.5 T and 3.0 T. Invest Radiol. 2018;53(5):257–63.
Nguyen KL, Park EA, Yoshida T, Hu P, Finn JP. Ferumoxytol enhanced black-blood cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2017;19(1):106.
Wu W, Klockow JL, Mohanty S, Ku KS, Aghighi M, Melemenidis S, et al. Theranostic nanoparticles enhance the response of glioblastomas to radiation. Nanotheranostics. 2019;3(4):299–310.
Prince MR, Zhang HL, Chabra SG, Jacobs P, Wang Y. A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J Xray Sci Technol. 2003;11(4):231–40.
Stirrat CG, et al. Ferumoxytol-enhanced magnetic resonance imaging methodology and normal values at 1.5 and 3T. J Cardiovasc Magn Reson. 2016;18(1):1–9.
Maki JH, et al. Dark blood magnetic resonance lymphangiography using dual-agent relaxivity contrast (DARC-MRL): a novel method combining gadolinium and iron contrast agents. Curr Probl Diagn Radiol. 2016;45(3):174–9.
Mitsumori LM, et al. Peripheral magnetic resonance lymphangiography: techniques and applications. Tech Vasc Interv Radiol. 2016;19(4):262–72.
Datta S, Satten G. A signed-rank test for clustered data. Biometrics. 2008;64:501–7.
Durkalski V, Palesch Y, Lipsitz S, Rust P. Analysis of clustered matched pair data. Stat Med. 2003;22:2417–28.
Gregg M. Marginal methods and software for clustered data with cluster- and group-size informativeness. PhD dissertation, University of Louisville, 2020.
Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61:29–48.
Gregg M, Datta S, Lorenz D. htestClust: reweighted marginal hypothesis tests for clustered data. R package version 0.2.0. https://CRAN.R-project.org/package=htestClust. 2020.
Nadler SBB, Hidalgo JHH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–32.
Data on file. AMAG Pharmaceuticals, Inc. https://www.feraheme.com/. Accessed 4 May 2021.
US Food and Drug Administration. FDA drug safety communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol). Accessed on March 19, 2021. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm440138.htm.
Wise-Faberowski L, Velasquez N, Chan F, Vasanawala S, McElhinney DB, Ramamoorthy C. Safety of ferumoxytol in children undergoing cardiac MRI under general anaesthesia. Cardiol Young. 2018;28(7):916–21.
Nguyen KL, Yoshida T, Kathuria-Prakash N, et al. Multicenter safety and practice for off-label diagnostic use of ferumoxytol in MRI. Radiology. 2019;293(3):554–64.
Neuwelt EA, Hamilton BE, Varallyay CG, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009;75(5):465–74.
Acknowledgements
The authors would like to thank Drs. John Carrino and Steven P. Daniels for their motivation for the project and support.
Hospital for Special Surgery has an institutional research agreement with General Electric Healthcare.
Funding
This study was funded by a Seed Grant from the International Skeletal Society (ISS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Disclaimer
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the ISS.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Queler, S.C., Tan, E.T., Geannette, C. et al. Ferumoxytol-enhanced vascular suppression in magnetic resonance neurography. Skeletal Radiol 50, 2255–2266 (2021). https://doi.org/10.1007/s00256-021-03804-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03804-w