Abstract
Objective
Determine if differences in T1ρ would be detected in specific regions or layers of patellofemoral cartilage between patients with symptomatic patellofemoral pain syndrome and asymptomatic control subjects.
Materials and methods
Ten subjects diagnosed with patellofemoral pain syndrome were compared with ten age-, gender-, and BMI-matched control subjects with no knee pain or prior trauma. Conventional turbo (fast) spin echo sequences and T1ρ-weighted imaging were performed on the symptomatic knee in each of the ten subjects. At the patella and distal femur, cartilage regions of interest were divided into medial and lateral sub-regions, each then further sub-divided by layer (superficial, middle, or deep). Two-tailed t test and chi-squared tests were used to analyze demographic data. A mixed effect model was run for each sub-region of T1ρ imaging. Statistical significance was determined using the likelihood ratio test against reduced models without patellofemoral pain syndrome symptomatic status as a fixed effect.
Results
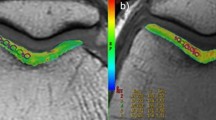
There was no difference in age, sex, or BMI between symptomatic and control patients. T1ρ values were significantly higher among patellofemoral pain syndrome patients when compared with controls in the superficial zone of the lateral patella (58.43 vs. 50.83, p = 0.03) and the middle zone of the lateral patella (52.67 vs. 43.60, p = 0.03). T1ρ was also higher in the superficial zone of the medial femur (50.94 vs. 46.70, p = 0.09) with a value approaching statistical significance.
Conclusion
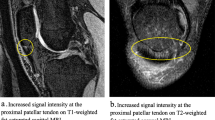
We report statistically significant differences in the T1ρ value in the superficial and middle zones of the lateral patella in patients with patellofemoral pain syndrome who had no abnormalities seen on conventional MRI sequences, suggesting an alteration the macromolecular structure of the cartilage in this population.


Similar content being viewed by others
References
Regatte RR, Akella SVS, Borthakur A, Reddy R. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J Magn Reson Imaging [Internet]. 2003 [cited 2019 May 31];17:114–21. Available from: http://doi.wiley.com/10.1002/jmri.10228.
Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med [Internet]. 1997 [cited 2019 May 31];38:863–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9402184.
Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med [Internet]. 2001 [cited 2019 May 31];46:419–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11550230.
Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1ρ MRI in articular cartilage systems. Magn Reson Med [Internet]. John Wiley & Sons, Ltd; 2004 [cited 2020 Feb 9];51:503–9. Available from: http://doi.wiley.com/10.1002/mrm.10710
Van Tiel J, Kotek G, Reijman M, Bos PK, Bron EE, Klein S, et al. Is T1r mapping an alternative to delayed gadolinium-enhanced mr imaging of cartilage in the assessment of sulphated glycosaminoglycan content in human osteoarthritic knees? An in vivo validation study. Radiology. Radiological Society of North America Inc. 2016;279:523–31.
Duvvuri U, Kudchodkar S, Reddy R, Leigh JS. T1rho relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthr Cartil. 2002;10:838–44.
Reddy R, Insko EK, Noyszewski EA, Dandora R, Kneeland JB, Leigh JS. Sodium MRI of human articular cartilage in vivo. Magn Reson Med [Internet]. 1998 [cited 2019 May 31];39:697–701. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9581599.
Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med [Internet]. 2001 [cited 2019 May 31];45:36–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11146483.
Eagle S, Potter HG, Koff MF. Morphologic and quantitative magnetic resonance imaging of knee articular cartilage for the assessment of post-traumatic osteoarthritis. J Orthop Res [Internet]. 2017 [cited 2020 Feb 5];35:412–23. Available from: http://doi.wiley.com/10.1002/jor.23345.
Witvrouw E, Callaghan MJ, Stefanik JJ, Noehren B, Bazett-Jones DM, Willson JD, et al. Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. Br J Sports Med [Internet]. 2014 [cited 2019 May 31];48:411–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24569145.
Stefanik JJ, Zhu Y, Zumwalt AC, Gross KD, Clancy M, Lynch JA, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the multicenter osteoarthritis study. Arthritis Care Res (Hoboken) [Internet]. 2010 [cited 2019 May 31];62:1258–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20506169.
Atkinson HF, Birmingham TB, Moyer RF, Yacoub D, Kanko LE, Bryant DM, et al. MRI T2 and T1ρ relaxation in patients at risk for knee osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord [Internet]. 2019 [cited 2020 Feb 5];20:182. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31039785.
Link TM, Li X. Establishing compositional MRI of cartilage as a biomarker for clinical practice. Osteoarthr Cartil [Internet]. 2018 [cited 2020 Feb 5];26:1137–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29550402.
Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr Cartil [Internet]. 2013 [cited 2020 Feb 5];21:1474–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23896316.
Regatte RR, Akella SVS, Borthakur A, Kneeland JB, Reddy R. In vivo proton MR three-dimensional t1ρ mapping of human articular cartilage: initial experience. Radiology [Internet]. 2003 [cited 2020 Feb 5];229:269–74. Available from: http://pubs.rsna.org/doi/10.1148/radiol.2291021041.
Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S. In vivo 3T spiral imaging based multi-slice T1ρ mapping of knee cartilage in osteoarthritis. Magn Reson Med [Internet]. 2005 [cited 2020 Feb 5];54:929–36. Available from: http://doi.wiley.com/10.1002/mrm.20609.
Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1ρ MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed [Internet]. 2006 [cited 2020 Feb 5];19:781–821. Available from: http://doi.wiley.com/10.1002/nbm.1102.
Nemeth A, Di Marco L, Boutitie F, Sdika M, Grenier D, Rabilloud M, et al. Reproducibility of in vivo magnetic resonance imaging T1rho and T2 relaxation time measurements of hip cartilage at 3.0T in healthy volunteers. J Magn Reson Imaging. John Wiley and Sons Inc. 2018;47:1022–33.
Thuillier DU, Souza RB, Wu S, Luke A, Li X, Feeley BT. T1ρ imaging demonstrates early changes in the lateral patella in patients with patellofemoral pain and maltracking. Am J Sports Med [Internet]. 2013 [cited 2019 May 31];41:1813–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23845401.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Source J R Stat Soc Ser B. 1995.
Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res [Internet]. 2004 [cited 2019 May 31];17–26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15232421.
Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev [Internet]. 2005 [cited 2019 May 31];33:195–200. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16239837.
Nugent GE, Schmidt TA, Schumacher BL, Voegtline MS, Bae WC, Jadin KD, et al. Static and dynamic compression regulate cartilage metabolism of PRoteoGlycan 4 (PRG4). Biorheology [Internet]. [cited 2019 May 31];43:191–200. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16912393.
Poole RA, Guilak F AS. Osteoarthritis: diagnosis and medical/surgical management [Internet]. 4th ed. In: Moskowitz RW, Altman RD, Hochberg MC, Buckwalter JA GV, editors. Philadelphia: Lippincott Williams & Wilkins; 2007 [cited 2019 May 31]. Available from: https://books.google.com/books?id=YfFj8Gbq5H0C&pg=PA27&source=gbs_toc_r&cad=3#v=onepage&q&f=false.
Fukui N, Miyamoto Y, Nakajima M, Ikeda Y, Hikita A, Furukawa H, et al. Zonal gene expression of chondrocytes in osteoarthritic cartilage. Arthritis Rheum [Internet]. 2008 [cited 2019 May 31];58:3843–53. Available from: http://doi.wiley.com/10.1002/art.24036
Lotz MK, Otsuki S, Grogan SP, Sah R, Terkeltaub R, D’Lima D. Cartilage cell clusters. Arthritis Rheum [Internet]. 2010 [cited 2019 may 31];62:2206–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20506158.
Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest [Internet]. 1995 [cited 2019 May 31];96:2859–69. Available from: http://www.jci.org/articles/view/118357.
Pedoia V, Gallo MC, Souza RB, Majumdar S. Longitudinal study using voxel-based relaxometry: association between cartilage T1ρ and T2 and patient reported outcome changes in hip osteoarthritis. J Magn Reson Imaging. John Wiley and Sons Inc. 2017;45:1523–33.
Wyatt C, Kumar D, Subburaj K, Lee S, Nardo L, Narayanan D, et al. Cartilage T1ρ and T2 relaxation times in patients with mild-to-moderate radiographic hip osteoarthritis. Arthritis Rheum. John Wiley and Sons Inc. 2015;67:1548–56.
Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, et al. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology Radiological Society of North America. 2011;258:832–42.
Li X, Wyatt C, Rivoire J, Han E, Chen W, Schooler J, et al. Simultaneous acquisition of T1ρ and T2 quantification in knee cartilage: repeatability and diurnal variation. J Magn Reson Imaging. John Wiley and Sons Inc. 2014;39:1287–93.
Acknowledgments
The authors would like to thank Dr. Hongzhe Li (Lee) , for his statistical assistance throughout the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human subjects were in accordance with the ethical standards or institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zelenski, N., Falk, D., D’Aquilla, K. et al. Zone- and layer-specific differences in proteoglycan content in patellofemoral pain syndrome are detectable on T1ρ MRI. Skeletal Radiol 49, 1397–1402 (2020). https://doi.org/10.1007/s00256-020-03418-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03418-8