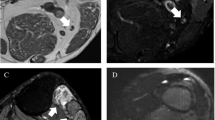
Abstract
Objective
To determine the utility of intravenous contrast in magnetic resonance neurography (MRN).
Materials and methods
A search of our PACS for MRN studies performed in 2015 yielded 74 MRN exams, 57 of which included pre- and post-contrast images. All studies were independently reviewed by 3 musculoskeletal radiologists with peripheral nerve imaging experience for presence/absence of nerve pathology, presence/absence of muscle denervation, and contrast utility score based on a 4-point Likert scale. The medical record was reviewed for demographic and clinical data.
Results
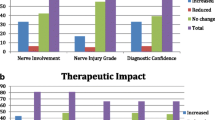
The mean contrast utility score across all readers and all cases was 1.65, where a score of 1 indicated no additional information and a score of 2 indicated mild additional information/supports interpretation. The mean contrast utility score was slightly higher in cases with a clinical indication of amputation/stump neuroma or mass (2.3 and 2.1 respectively) and lower in cases with a clinical indication of trauma (1.5). The mean contrast utility score was lowest in patients undergoing MRN for pain, numbness, and/or weakness (1.2).
Conclusion
Intravenous contrast provides mild to no additional information for the majority of MRN exams. Given the invasive nature of contrast and recent concerns regarding previously unrecognized risks of repetitive contrast exposure, assessment of the necessity of intravenous contrast in MRN is important. Consensus evidence-based practice guidelines regarding intravenous contrast use in MRN are necessary.






Similar content being viewed by others
References
Howe FA, Filler AG, Bell BA, Griffiths JR. Magnetic resonance neurography. Magn Reson Med. 1992;28(2):328–38.
Chhabra A, Williams EH, Wang KC, Dellon AL, Carrino JA. MR neurography of neuromas related to nerve injury and entrapment with surgical correlation. AJNR Am J Neuroradiol. 2010;31:1363–8.
Thawait SK, Chaudhry V, Thawait GK, et al. High-resolution MR neurography of diffuse peripheral nerve lesions. AJNR Am J Neuroradiol. 2011;32(8):1365–72.
Andreisek G, Crook DW, Burg D, Marincek B, Weishaupt D. Peripheral neuropathies of the median, radial and ulnar nerves: MR imaging features. RadioGraphics. 2006;26:1267–87.
Andreisek G, Burg D, Studer A, Weishaupt D. Upper extremity peripheral neuropathies: role and impact of MR imaging on patient management. Eur Radiol. 2008;18:1953–61.
Andreisek G, Chhabra A. MR neurography: pitfalls in imaging and interpretation. Semin Musculoskelet Radiol. 2015;19(2):94–102.
Chazen JL, Cornman-homonoff J, Zhao Y, Sein M, Feuer N. MR neurography of the lumbosacral plexus for lower extremity radiculopathy: frequency of findings, characteristics of abnormal Intraneural signal, and correlation with electromyography. AJNR Am J Neuroradiol. 2018;39(11):2154–60.
Chhabra A. Peripheral MR neurography: approach to interpretation. Neuroimaging Clin N Am. 2014;24(1):79–89.
Chhabra A, Andreisek G, Soldatos T, et al. MR neurography: past, present, and future. AJR. 2011;197(3):583–91.
Chhabra A, Zhao L, Carrino JA, et al. MR neurography: advances. Radiol Res Pract. 2013;2013:809568.
Chhabra A, Carrino J. Current MR neurography techniques and whole-body MR neurography. Semin Musculoskelet Radiol. 2015;19(2):79–85.
Chhabra A, Bajaj G, Wadhwa V, et al. MR neurographic evaluation of facial and neck pain: normal and abnormal craniospinal nerves below the skull base. Radiographics. 2018;38(5):1498–513.
Chhabra A, Madhuranthakam AJ, Andreisek G. Magnetic resonance neurography: current perspectives and literature review. Eur Radiol. 2018;28(2):698–707.
Daniels SP, Feinberg JH, Carrino JA, Behzadi AH, Sneag DB. MRI of foot drop: how we do it. Radiology. 2018;289(1):9–24.
Deshmukh S, Carrino JA, Feinberg JH, Wolfe SW, Eagle S, Sneag DB. Pins and needles from fingers to toes: high-resolution MRI of peripheral sensory mononeuropathies. AJR Am J Roentgenol. 2017;208(1):W1–W10.
Deshmukh S, Fayad LM, Ahlawat S. MR neurography (MRN) of the long thoracic nerve: retrospective review of clinical findings and imaging results at our institution over 4 years. Skelet Radiol. 2017;46(11):1531–40.
Deshmukh SD, Samet J, Fayad LM, Ahlawat S. Magnetic resonance neurography of traumatic pediatric peripheral nerve injury: beyond birth-related brachial palsy. Pediatr Radiol. 2019;49(7):954–64.
Petchprapa CN, Rosenberg ZS, Sconfienza LM, Cavalcanti CF, Vieira RL, Zember JS. MR imaging of entrapment neuropathies of the lower extremity. Part 1. The pelvis and hip. RadioGraphics. 2010;30:983–1000.
Blumfield E, Swenson DW, Iyer RS, et al. Gadolinium-based contrast agents – review of recent literature on magnetic resonance imaging signal intensity changes and tissue deposits, which emphasis on pediatric patients. Pediatr Radiol. 2019;49:448.
Boyken J, Frenzel T, Lohrke J, Jost G, Pietsch H. Gadolinium accumulation in the deep cerebellar nuclei and globus pallidus after exposure to linear but not macrocyclic gadolinium-based contrast agents in a retrospective pig study with high similarity to clinical conditions. Investig Radiol. 2018;53(5):278–85.
Kanal E. Gadolinium-based contrast agents: the plot thickens. Radiology. 2017;285(2):340–2.
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834–41.
Levine D, Mcdonald RJ, Kressel HY. Gadolinium retention after contrast-enhanced MRI. JAMA. 2018;320(18):1853–4.
Lord ML, Chettle DR, Gräfe JL, Noseworthy MD, Mcneill FE. Observed deposition of gadolinium in bone using a new noninvasive in vivo biomedical device: results of a small pilot feasibility study. Radiology. 2018;287(1):96–103.
Pinter NK, Klein JP, Mechtler LL. Potential safety issues related to the use of gadolinium-based contrast agents. Continuum (Minneap Minn). 2016;22(5, Neuroimaging):1678–84.
Ramalho J, Castillo M, Alobaidy M, et al. High signal intensity in Globus Pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology. 2015;276(3):836–44.
Ramalho J, Semelka RC, Ramalho M, Nunes RH, Alobaidy M, Castillo M. Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol. 2016;37(7):1192–8.
Thomsen HS, Morcos SK, Almén T, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR contrast medium safety committee guidelines. Eur Radiol. 2013;23(2):307–18.
Wang L, Niu Y, Kong X, et al. The application of paramagnetic contrast-based T2 effect to 3D heavily T2W high-resolution MR imaging of the brachial plexus and its branches. Eur J Radiol. 2016;85(3):578–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Harrell, A.D., Johnson, D., Samet, J. et al. With or without? A retrospective analysis of intravenous contrast utility in magnetic resonance neurography. Skeletal Radiol 49, 577–584 (2020). https://doi.org/10.1007/s00256-019-03321-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03321-x