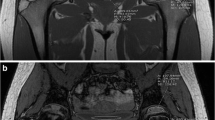
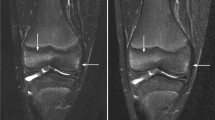
Abstract
Objective
To determine the threshold signal drop on 3-T chemical shift imaging (CSI), with in-phase (IP) and opposed-phase (OP) sequences, for accurately identifying bone marrow replacement with 100% sensitivity, and determine a clinically useful measurement method for deriving such a threshold.
Materials and methods
From a convenience series of 157 MRIs, 36 cases with histologically proven marrow-replacing lesions and 22 sites of red marrow (histologically proven (2) or with minimum 6-month stability) with 3-Tesla CSI were included. Two musculoskeletal radiologists performed two measurement methods (first: multiple algorithmic ROIs at the top, middle, and bottom of lesions (M-ROI); second: an ROI was drawn where there appeared to be the least opposed-phase signal reduction qualitatively/visually (Q-ROI)). Lesional and red marrow signal change (%,[(IP-OP)signal/IP signal]*100) was determined. Statistical analyses included Student’s t test, Cohen’s kappa, and receiver operator characteristic curve generation.
Results
By M-ROI, lesion signal change was − 0.508% (confidence interval (CI) = − 5.537:4.521) and 1.348% (CI = − 3.541:6.311) for readers 1 and 2. By Q-ROI, lesion signal change was − 11.03% (CI = − 17.01:- 5.046) and − 5.657% (CI = − 12.36:1.048) for readers 1 and 2. For all M-ROI and Q-ROI measurement strategies, signal change between lesional tissue and red marrow was significantly different (p < 0.0001). QROI produced the best composite sensitivities and specificities with a maximized Youden index of 0.955–1. A threshold signal drop of 25% with Q-ROI produced at least 100%/86% sensitivity/specificity for both readers for identifying marrow replacement.
Conclusions
For 3-T CSI, a single visually targeted measurement using a 25% threshold is accurate for identifying marrow-replacing lesions.







Similar content being viewed by others
References
Fayad LM, Jacobs MA, Wang X, Carrino JA, Bluemke DA. Musculoskeletal tumors: how to use anatomic, functional, and metabolic MR techniques. Radiology. 2012;265:340–56.
Richardson ML. Bone marrow abnormalities revealed by MR imaging. AJR Am J Roentgenol. 1998;171:261–2.
Richardson ML. Optimizing pulse sequences for magnetic resonance imaging of the musculoskeletal system. Radiol Clin N Am. 1986;24:137–44.
Vande Berg BC, Malghem J, Lecouvet FE, et al. Magnetic resonance imaging of normal bone marrow. Eur Radiol. 1998;8:1327–34.
Richardson ML, Amparo EG, Gillespy T, Helms CA, Demas BE, Genant HK. Theoretical considerations for optimizing intensity differences between primary musculoskeletal tumors and normal tissue with spin-echo magnetic resonance imaging. Investig Radiol. 1985;20:492–7.
Shiga NT, Del Grande F, Lardo O, Fayad LM. Imaging of primary bone tumors: determination of tumor extent by non-contrast sequences. Pediatr Radiol. 2013;43:1017–23.
Zajick DC Jr, Morrison WB, Schweitzer ME, Parellada JA, Carrino JA. Benign and malignant processes: normal values and differentiation with chemical shift MR imaging in vertebral marrow. Radiology. 2005;237:590–6.
Amin WM, Kotb HT, Abdel-Kerim AA, Barakat MS, El-Malky AA, Fadel SH. Diffusion-weighted MRI and in-phase/opposed-phase sequences in the assessment of bone tumors. J Magn Reson Imaging. 2016;44:565–72.
Disler DG, McCauley TR, Ratner LM, Kesack CD, Cooper JA. In-phase and out-of-phase MR imaging of bone marrow: prediction of neoplasia based on the detection of coexistent fat and water. AJR Am J Roentgenol. 1997;169:1439–47.
Del Grande F, Subhawong T, Flammang A, Fayad LM. Chemical shift imaging at 3 Tesla: effect of echo time on assessing bone marrow abnormalities. Skelet Radiol. 2014;43:1139–47.
Del Grande F, Tatizawa-Shiga N, Jalali Farahani S, Chalian M, Fayad LM. Chemical shift imaging: preliminary experience as an alternative sequence for defining the extent of a bone tumor. Quant Imaging Med Surg. 2014;4:173–80.
Dreizin D, Ahlawat S, Del Grande F, Fayad LM. Gradient-echo in-phase and opposed-phase chemical shift imaging: role in evaluating bone marrow. Clin Radiol. 2014;69:648–57.
Erly WK, Oh ES, Outwater EK. The utility of in-phase/opposed-phase imaging in differentiating malignancy from acute benign compression fractures of the spine. AJNR Am J Neuroradiol. 2006;27:1183–8.
Hajek PC, Baker LL, Goobar JE, Sartoris DJ, Hesselink JR, Haghighi P, et al. Focal fat deposition in transverse bone marrow: MR characteristics. Radiology. 1987;162:245–9.
Zampa V, Cosottini M, Michelassi C, Ortori S, Bruschini L, Bartolozzi C. Value of opposed-phase gradient-echo technique in distinguishing between benign and malignant vertebral lesions. Eur Radiol. 2002;12:1811–8.
Winfeld M, Ahlawat S, Safdar N. Utilization of chemical shift MRI in the diagnosis of disorders affecting pediatric bone marrow. Skelet Radiol. 2016;45:1205–12.
Parizel PM, Van Riet B, Van Hasselt BA, Van Goethem JW, Van Den Hauwe L, Dijkstra HA, et al. Influence of magnetic field strength on T2* decay and phase effects in gradient echo MRI of vertebral bone marrow. J Comput Assist Tomogr. 1995;19(3):465–71.
Li G, Xu Z, Li X, Yuan W. Comparison of chemical shift-encoded water–fat MRI and MR spectroscopy in quantification of marrow fat in postmenopausal females. J Magn Reson Imaging. 2017;45(1):66–73.
Carroll KW, Feller JF, Tirman PF. Useful internal standards for distinguishing infiltrative marrow pathology from hematopoietic marrow at MRI. J Magn Reson Imaging. 1997;7(2):394–8.
Hajek PC, Baker LL, Goobar JE, Sartoris DJ, Hesselink JR, Haghighi P, et al. Focal fat deposition in transverse bone marrow: MR characteristics. Radiology. 1987;162:245–9.
De Bazelaire CMJ, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004;230:652–9.
Karampinos DC, Melkus G, Baum T, Bauer JS, Rummeny EJ, Krug R. Bone marrow fat quantification in the presence of trabecular bone: initial comparison between water-fat imaging and single-voxel MRS. Magn Reson Med. 2014;71(3):1158–65.
Ojanen X, Borra RJ, Havu M, et al. Comparison of vertebral bone marrow fat assessed by 1H MRS and in-phase and out-of-phase MRI among family members. Osteoporos Int. 2014;25:653–62.
Sims RD, Yuan Q, Khatri G, Weatherall PT, Batz R, Zhang S, Pedrosa I, Rofsky NM. Multiecho 2-point Dixon (mDIXON) imaging as an alternative to separate 2D chemical shift imaging and 3D fat-suppressed T1-weighted sequences for gadolinium enhanced imaging. Proc Intl Soc Mag Reson Med 20;2012.
Ahlawat S, Khandheria P, Del Grande F, Morelli J, Subhawong TK, Demehri S, et al. Interobserver variability of selective region-of-interest measurement protocols for quantitative diffusion weighted imaging in soft tissue masses: comparison with whole tumor volume measurements. J Magn Reson Imaging. 2016;43:446–54.
Hanrahan CJ, Christensen CR, Crim JR. Current concepts in the evaluation of multiple myeloma with MR imaging and FDG PET/CT. Radiographics. 2010;30(1):127–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Grant support
None.
Rights and permissions
About this article
Cite this article
Kumar, N.M., Ahlawat, S. & Fayad, L.M. Chemical shift imaging with in-phase and opposed-phase sequences at 3 T: what is the optimal threshold, measurement method, and diagnostic accuracy for characterizing marrow signal abnormalities?. Skeletal Radiol 47, 1661–1671 (2018). https://doi.org/10.1007/s00256-018-2999-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-018-2999-0