Abstract
Objective
The purpose of this study was to evaluate differences in parameters of diffusion kurtosis imaging (DKI) and minimum apparent diffusion coefficient (ADCmin) between benign and malignant musculoskeletal tumors.
Materials and methods
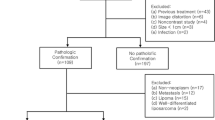
In this prospective study, 43 patients were scanned using a DKI protocol on a 3-T MR scanner. Eligibility criteria were: non-fatty, non-cystic soft tissue or osteolytic tumors; > 2 cm; location in the retroperitoneum, pelvis, leg, or neck; and no prior treatment. They were clinically or histologically diagnosed as benign (n = 27) or malignant (n = 16). In the DKI protocol, diffusion-weighted imaging was performed using four b values (0-2000 s/mm2) and 21 diffusion directions. Mean kurtosis (MK) values were calculated on the MR console. A recently developed software application enabling reliable calculation was used for DKI analysis.
Results
MK showed a strong correction with ADCmin (Spearman’s rs = 0.95). Both MK and ADCmin values differed between benign and malignant tumors (p < 0.01). For benign and malignant tumors, the mean MK values (± SD) were 0.49 ± 0.17 and 1.14 ± 0.30, respectively, and ADCmin values were 1.54 ± 0.47 and 0.49 ± 0.17 × 10−3 mm2/s, respectively. At cutoffs of MK = 0.81 and ADCmin = 0.77 × 10−3 mm2/s, the specificity and sensitivity for diagnosis of malignant tumors were 96.3 and 93.8% for MK and 96.3 and 93.8% for ADCmin, respectively. The areas under the curve were 0.97 and 0.99 for MK and ADCmin, respectively (p = 0.31).
Conclusions
MK and ADCmin showed high diagnostic accuracy and strong correlation, reflecting the accuracy of MK. However, no clear added value of DKI could be demonstrated in differentiating musculoskeletal tumors.




Similar content being viewed by others
References
Surov A, Nagata S, Razek AA, Tirumani SH, Wienke A, Kahn T. Comparison of ADC values in different malignancies of the skeletal musculature: a multicentric analysis. Skelet Radiol. 2015;44(7):995–1000.
Teixeira PA, Gay F, Chen B, Zins M, Sirveaux F, Felblinger J, et al. Diffusion-weighted magnetic resonance imaging for the initial characterization of non-fatty soft tissue tumors: correlation between T2 signal intensity and ADC values. Skelet Radiol. 2016;45(2):263–71.
Ahlawat S, Khandheria P, Subhawong TK, Fayad LM. Differentiation of benign and malignant skeletal lesions with quantitative diffusion weighted MRI at 3T. Eur J Radiol. 2015;84(6):1091–7.
Surov A, Behrmann C. Diffusion-weighted imaging of skeletal muscle lymphoma. Skelet Radiol. 2014;43(7):899–903.
Karchevsky M, Babb JS, Schweitzer ME. Can diffusion-weighted imaging be used to differentiate benign from pathologic fractures? A meta-analysis. Skelet Radiol. 2008;37(9):791–5.
Jiang R, Jiang J, Zhao L, Zhang J, Zhang S, Yao Y, et al. Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget. 2015;6(39):42380–93.
Tonoyan AS, Pronin IN, Pitshelauri DI, Shishkina LV, Fadeeva LM, Pogosbekyan EL, et al. A correlation between diffusion kurtosis imaging and the proliferative activity of brain glioma. Zh Vopr Neirokhir Im N N Burdenko. 2015;79(6):5–14.
Yuan J, Yeung DK, Mok GS, Bhatia KS, Wang YX, Ahuja AT, et al. Non-Gaussian analysis of diffusion weighted imaging in head and neck at 3T: a pilot study in patients with nasopharyngeal carcinoma. PLoS One. 2014;9(1):e87024.
Tamura C, Shinmoto H, Soga S, Okamura T, Sato H, Okuaki T, et al. Diffusion kurtosis imaging study of prostate cancer: preliminary findings. J Magn Reson Imaging. 2014;40(3):723–9.
Wu D, Li G, Zhang J, Chang S, Hu J, Dai Y. Characterization of breast tumors using diffusion kurtosis imaging (DKI). PLoS One. 2014;9(11):e113240.
Hu F, Tang W, Sun Y, Wan D, Cai S, Zhang Z, et al. The value of diffusion kurtosis imaging in assessing pathological complete response to neoadjuvant chemoradiation therapy in rectal cancer: a comparison with conventional diffusion-weighted imaging. Oncotarget. 2017.
Hori M, Tsutsumi S, Yasumoto Y, Ito M, Suzuki M, Tanaka FS, et al. Cervical spondylosis: evaluation of microstructural changes in spinal cord white matter and gray matter by diffusional kurtosis imaging. Magn Reson Imaging. 2014;32(5):428–32.
Glenn GR, Tabesh A, Jensen JH. A simple noise correction scheme for diffusional kurtosis imaging. Magn Reson Imaging. 2015;33(1):124–33.
Yokosawa S, Sasaki M, Bito Y, Ito K, Yamashita F, Goodwin J, et al. Optimization of scan parameters to reduce acquisition time for diffusion kurtosis imaging at 1.5T. Magn Reson Med Sci. 2016;15(1):41–8.
Yokosawa S, Ochi S, Bito Y, Ito K, Sasaki M. Robust estimation with suppressed image blurring for diffusion kurtosis imaging using selective spatial smoothing filter. Proc Intl Soc Magn Reson Med. 2014;2581.
Motulsky H. Appropriate simple size. In: Motulsky H, editor. Trans intuitive biostatics. Tokyo: Science Medical; 1997. p. 196–205.
Oka K, Yakushiji T, Sato H, Hirai T, Yamashita Y, Mizuta H. The value of diffusion-weighted imaging for monitoring the chemotherapeutic response of osteosarcoma: a comparison between average apparent diffusion coefficient and minimum apparent diffusion coefficient. Skelet Radiol. 2010;39(2):141–6.
Baliyan V, Das CJ, Sharma R, Gupta AK. Diffusion-weighted imaging: technique and applications. World J Radiol. 2016;8(9):785–98.
Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. NeuroImage. 2012;59(1):467–77.
Zhu J, Zhuo C, Qin W, Wang D, Ma X, Zhou Y, et al. Performances of diffusion kurtosis imaging and diffusion tensor imaging in detecting white matter abnormality in schizophrenia. Neuroimage Clin. 2015;7:170–6.
Nagata S, Nishimura H, Uchida M, Sakoda J, Tonan T, Hiraoka K, et al. Diffusion-weighted imaging of soft tissue tumors: usefulness of the apparent diffusion coefficient for differential diagnosis. Radiat Med. 2008;26(5):287–95.
Douis H, Jeys L, Grimer R, Vaiyapuri S, Davies AM. Is there a role for diffusion-weighted MRI (DWI) in the diagnosis of central cartilage tumors? Skelet Radiol. 2015;44(7):963–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no financial or other conflicts of interest in relation to this paper.
Rights and permissions
About this article
Cite this article
Ogawa, M., Kan, H., Arai, N. et al. Differentiation between malignant and benign musculoskeletal tumors using diffusion kurtosis imaging. Skeletal Radiol 48, 285–292 (2019). https://doi.org/10.1007/s00256-018-2946-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-018-2946-0