Abstract
Objective
There is a close relation between cartilage health and its hydration state. Current magnetic resonance methods allow visualizing this tissue. However, a quantitative analysis is more useful when studying disease. The purpose of this study was to quantify water content in cartilage using magnetic resonance without contrast agents.
Materials and methods
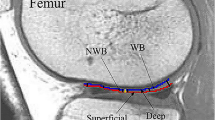
Water-content estimations using T1 magnetic resonance mapping were done first in eight gelatin samples where the water content was previously known. The same method was used in the physeal areas of eight skeletally immature 30-kg pigs. To calculate accuracy, T1 calculations were compared to dry-freeze, which is considered the gold standard because it can remove the total water content form a tissue. Four fresh cartilage and seven gelatin samples were dry-frozen. Water content obtained from dry-freeze was compared to the one calculated from T1 map values. A mathematical model and statistical analysis were used to calculate the predictive value of the method and its significance.
Results
T1-map-based magnetic resonance method can calculate water content in cartilage with an accuracy of 97.3 %. We calculated a coefficient of variance for this method against dry-frozen sample of 3.68 (SD = 1.2) in gelatin samples, and 2.73 (SD = 1.3) in in vivo samples. Between two independent observers, the coefficient of variance was 0.053, which suggests it can be easily reproduced.
Conclusions
Magnetic resonance was able to calculate, with high accuracy, the cartilage water content using T1 mapping sequences.








Similar content being viewed by others
References
Li X, Wang R, Li Y, Tang L, Xu A, Hu J. MRI characteristics and transverse relaxation time measurements in normal growing cartilage. J Huazhong Univ Sci Technolog Med Sci. 2004;24(4):411–3.
Jazrawi ML, Alaia MJ, Chang G, FitzGerald EF, Recht MP. Advances in magnetic resonance imaging of articular cartilage. JAAOS. 2011;19(7):420–9.
Winalski CS, Rajiah P. The evolution of articular cartilage imaging and its impact on clinical practice. Skeletal Radiol. 2011;40(9):1197–222.
Damion RA, Pawaskar SS, Ries ME, Ingham E, Williams S, Jin Z, et al. Spin–lattice relaxation rates and water content of freeze-dried articular cartilage. Osteoarthr Cartil. 2012;20(2):184–90.
Buckland-Wright JC, Macfarlane DG, Lynch JA, Jasani MK, Bradshaw CR. Joint space width measures cartilage thickness in osteoarthritis of the knee: high-resolution plain-film and double-contrast macroradiographic investigation. Ann Rheum Dis. 1995;54:263–8.
Staroswiecki E, Granlund KL, Alley MT, Gold GE, Hargreaves BA. Simultaneous estimation of T2 and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional Double Echo Steady State (DESS) sequence at 3T. Magn Reson Med. Forthcoming 2012. doi:10.1002/mrm.23090.
Yoo WJ, Kocher MS, Micheli LJ. Growth plate disturbance after transphyseal reconstruction of the anterior cruciate ligament in skeletally immature adolescent patients: an MR imaging study. J Pediatr Orthop. 2011;31(6):691–6.
Bekkers JE, Bartels LW, Vincken KL, Dhert WJ, Creemers LB, Saris DB. Articular cartilage evaluation after TruFit plug implantation analyzed by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC). Am J Sports Med. 2013. doi:10.1177/0363546513483536 [Epub ahead of print].
Bekkers JE, Bartels LW, Benik RJ, Tsuchida AI, Vincken KL, Dhert WJ, et al. Delayed gadolinium-enhanced MRI or cartilage (dGEMRIC) can be effectively applied for longitudinal cohort evaluation of articular cartilage regeneration. Osteoarthr Cartil. 2013;S1063–4584(13):758–9. doi:10.10167j.joca.2013.03.017.
Carstens A, Kirberger RM, Elleman M, Dahlberg LE, Fletcher L, Lammentausta E. Feasibility for mapping cartilage T1 relaxation times in the distal metacarpus3/metatarsus3 of thoroughbred racehorses using delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC): normal cadaver study. Vet Radiol Ultrasound. 2013. doi:10.1111/vru.12030 [Epub ahead or print].
Xie L, Lin ASP, Kundu K, Levenston ME, Murthy N, Guldberg RE. Quantitative imaging of cartilage and bone morphology, reactive oxygen species, and vascularization in a rodent model of osteoarthritis. Arthritis Rheum. Forthcoming 2012. doi:10.1002/art.34370.
Wardak E, Gill S, Wardak M, Sen R, Singh P, Kumar V, et al. Role of MRI in detecting early physeal changes due to acute osteoarticular infection around the knee joint: a pilot study. Int Orthop. 2009;33(6):1707–11.
Siepmann DB, McGovern JM, Gold GE, Brittain JH, Reeder SB. High-resolution 3D cartilage imaging of the knee at 3T in five minutes using IDEAL-SPGR and parallel imaging. Proc Intl Soc Mag Reson. 2006;14:1251.
Fatouros PP, Marmarou A, Kraft KA, Inao S, Schwarz FP. In vivo brain water determination by T1 measurements: effect of total water content, hydration fraction, and field strength. Magn Reson Med. 1991;17:402–13.
Venkatesan R, Lin W, Gurleyik K, He YY, Paczynski RP, Powers WJ, et al. Absolute measurements of water content using magnetic resonance imaging: preliminary findings in an in vivo focal ischemic rat model. Magn Reson Med. 2000;43:146–450.
Jennings TA. Lyophilization: introduction and basic principles. 1st ed. Interpharm/CRC;1999.
Braun HJ, Gold GE. Diagnosis of osteoarthritis: Imaging. Bone. Forthcoming 2012. doi:10.16/j.bone.2011.11.019.
Raya J, Horng A, Dietrich O, Krasnokutsky S, Beltran LS, Storey P, et al. Articular cartilage: in vivo diffusion-tensor imaging. Radiology. 2012;262(2):550–9.
Eckstein F, Wirth W. Quantitative cartilage imaging in knee osteoarthritis. Arthritis. 2010;2011:475–684. Epub.
Li X, Wang R, Qi J, Tang L. Gadolinium-enhanced MR imaging of epiphyseal and metaphyseal marrow in normal piglets. J Huazhong Univ Sci Technolog Med Sci. 2005;25(4):461–3.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiguetomi-Medina, J.M., Gottliebsen, M., Kristiansen, M.S. et al. Water-content calculation in growth plate and cartilage using MR T1-mapping design and validation of a new method in a porcine model. Skeletal Radiol 42, 1413–1419 (2013). https://doi.org/10.1007/s00256-013-1674-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-013-1674-8