Abstract
Objective
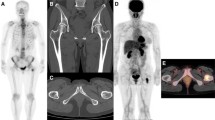
By analyzing bone scans we aimed to determine whether the assessment of the central skeleton is sufficient for osseous staging in breast cancer patients. This might be of interest for future staging modalities, especially positron emission tomography/computed tomography, usually sparing the peripheral extremities, as well as the skull.
Materials and methods
In this retrospective study, a total of 837 bone scans for initial staging or restaging of breast cancer were included. A total of 291 bone scans in 172 patients were positive for bone metastases. The localization and distribution of the metastases were re-evaluated by two readers in consensus. The extent of the central skeleton involvement was correlated to the incidence of peripheral metastases.
Results
In all 172 patients bone metastases were seen in the central skeleton (including the proximal third of humerus and femur). In 34 patients (19.8 %) peripheral metastases of the extremities (distally of the proximal third of humerus and femur) could be detected. Sixty-four patients (37.2 %) showed metastases of the skull. Summarizing the metastases of the distal extremities and skull, 79 patients (45.9 %) had peripheral metastases. None of the patients showed peripheral metastases without any affliction of the central skeleton. The incidence of peripheral metastases significantly correlated with the extent of central skeleton involvement (p < 0.001).
Conclusions
Regarding bone scans, an isolated metastatic spread to the peripheral skeleton without any manifestation in the central skeleton seems to be the exception. Thus, the assessment of the central skeleton should be sufficient in osseous breast cancer staging and restaging. However, in case of central metastases, additional imaging of the periphery should be considered for staging and restaging.

Similar content being viewed by others
References
Galasko CS. Skeletal metastases and mammary cancer. Ann R Coll Surg Engl. 1972;50:3–28.
Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–6.
Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336–40.
Ahmed A, Glynne-Jones R, Ell PJ. Skeletal scintigraphy in carcinoma of the breast—a ten-year retrospective study of 389 patients. Nucl Med Commun. 1990;11:421–6.
Wikenheiser KA, Silberstein EB. Bone scintigraphy screening in stage I-II breast cancer: is it cost-effective? Cleve Clin J Med. 1996;63:43–7.
Yip CH, Paramsothy M. Value of routine 99mTc-MDP bone scintigraphy in the detection of occult skeletal metastases in women with primary breast cancer. Breast. 1999;8:267–9.
Myers RE, Johnston M, Pritchard K, et al. Baseline staging tests in primary breast cancer: a practice guideline. CMAJ. 2001;164:1439–44.
Dose-Schwarz J, Mahner S, Schirrmacher S, et al. [Detection of metastases in breast cancer patients: comparison of FDG PET with chest X-ray, bone scintigraphy and ultrasound of the abdomen]. Nuklearmedizin. 2008;47:97–103 [in German].
Taira AV, Herfkens RJ, Gambhir SS, Quon A. Detection of bone metastases: assessment of integrated FDG PET/CT imaging. Radiology. 2007;243:204–11.
Ghanem N, Altehoefer C, Kelly T, et al. Whole-body MRI in comparison to skeletal scintigraphy in detection of skeletal metastases in patients with solid tumors. In Vivo. 2006;20:173–82.
Hahn S, Heusner T, Kummel S, et al. Comparison of FDG-PET/CT and bone scintigraphy for detection of bone metastases in breast cancer. Acta Radiol. 2011;52:1009–14.
Dose J, Bleckmann C, Bachmann S, et al. Comparison of fluorodeoxyglucose positron emission tomography and “conventional diagnostic procedures” for the detection of distant metastases in breast cancer patients. Nucl Med Commun. 2002;23:857–64.
Liu T, Cheng T, Xu W, et al. A meta-analysis of 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with breast cancer. Skeletal Radiol. 2011;40:523–31.
Hamaoka T, Madewell JE, Podoloff DA, et al. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–53.
Costelloe CM, Rohren EM, Madewell JE, et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606–14.
Shie P, Cardarelli R, Brandon D, et al. Meta-analysis: comparison of F-18 fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastases in patients with breast cancer. Clin Nucl Med. 2008;33:97–101.
Heusner TA, Kuemmel S, Hahn S, et al. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur J Nucl Med Mol Imaging. 2009;36:1543–50.
Hausmann D, Kern C, Schroder MT, et al. [Whole-body MRI in preoperative diagnostics of breast cancer–a comparison with [corrected] staging methods according to the S 3 guidelines]. Rofo. 2011;183:1130–7 [in German].
Du Y, Cullum I, Illidge TM, Ell PJ. Fusion of metabolic function and morphology: sequential [18F]fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. J Clin Oncol. 2007;25:3440–7.
Koolen BB, Vrancken Peeters MJ, Aukema TS, et al. 18F-FDG PET/CT as a staging procedure in primary stage II and III breast cancer: comparison with conventional imaging techniques. Breast Cancer Res Treat. 2012;131:117–26.
Riegger C, Herrmann J, Nagarajah J, et al. Whole-body FDG PET/CT is more accurate than conventional imaging for staging primary breast cancer patients. Eur J Nucl Med Mol Imaging. 2012;39(5):852–63
Delbeke D, Coleman RE, Guiberteau MJ, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–95.
Boxer DI, Todd CE, Coleman R, Fogelman I. Bone secondaries in breast cancer: the solitary metastasis. J Nucl Med. 1989;30:1318–20.
Koizumi M, Yoshimoto M, Kasumi F, Ogata E. Comparison between solitary and multiple skeletal metastatic lesions of breast cancer patients. Ann Oncol. 2003;14:1234–40.
Krasnow AZ, Hellman RS, Timins ME, et al. Diagnostic bone scanning in oncology. Semin Nucl Med. 1997;27:107–41.
Kardamakis D, Vassiliou V, Chow E. Pathophysiology of bone metastases. In: Kardamakis D, Vassiliou V, Chow E, editors. Bone metastases: A translational and clinical approach: 12 (cancer metastasis - biology and treatment). chapter 4.3. Netherlands: Springer; 2009. pp. 80–5.
Ribatti D, Mangialardi G, Vacca A. Stephen Paget and the ‘seed and soil’ theory of metastatic dissemination. Clin Exp Med. 2006;6:145–9.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krammer, J., Engel, D., Schnitzer, A. et al. Is the assessment of the central skeleton sufficient for osseous staging in breast cancer patients? A retrospective approach using bone scans. Skeletal Radiol 42, 787–791 (2013). https://doi.org/10.1007/s00256-012-1562-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-012-1562-7