Abstract
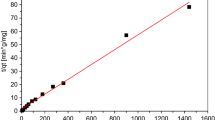
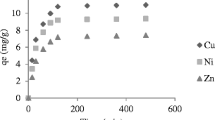
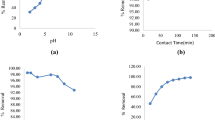
Oil shale is able to remove appreciable amounts of copper and zinc ions from aqueous solutions. It was noted that an increase in the adsorbent concentration with constant copper or zinc concentration resulted in greater metal removal from solution. An increase in the copper or zinc concentration with a constant sorbent concentration resulted in higher metal loading per unit weight of sorbent. For both metals, copper and zinc, equilibrium was attained after 24-h contact time. Increase in the initial pH or temperature of the metal solution resulted in an increase in the metal uptake per unit weight of the sorbent. Freundlich isotherm model was found to be applicable for the experimental data of Cu2+ and Zn2+. The results showed that oil shale could be used for the adsorption of the Cu2+ and Zn2+ with higher affinity toward Zn2+ ions. Addition of sodium salt to the metal solution influenced copper removal positively, but inhibited zinc removal.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 3 January 2000 · Accepted: 27 June 2000
Rights and permissions
About this article
Cite this article
Al-Asheh, S., Banat, F. Adsorption of copper and zinc by oil shale. Environmental Geology 40, 693–698 (2001). https://doi.org/10.1007/s002540000234
Issue Date:
DOI: https://doi.org/10.1007/s002540000234