Abstract
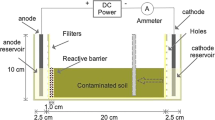
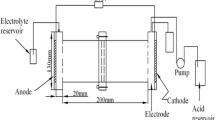
Wanshan mercury mine is the largest cinnabar deposit in Guizhou, China. Few effective methods had been achieved to remedy Hg heavily contaminated field soils. In this paper, a modified EK method with approaching cathodes (AC-EK) and an I−/I2 lixiviant was described to remedy mercury-contaminated field soils near Wanshan mercury mine. Paddy Soil I and Paddy Soil II were sampled and contained 576.73 ± 45.50 and 491.35 ± 4.73 mg/kg Hg, respectively. Although they contained 6.9 and 9.4% organic matter respectively, more than 92 and 89% Hg were removed by AC-EK within 5 days. Removal ratio increased by 0.21 and 0.68 times using EK process with ACs over that with one single cathode (SC-EK). AC-EK method saved nearly 26.4–28.1% electric power as compared to SC-EK method. As an I−/I2 lixiviant solution was used to solubilize HgS(HgO) during EK process, the bonding of Hg to organic functional S groups should be less important than the binding to inner sites of organic matter in soil. The relationship between EK remediation effect and soil organic matter content was fitted to a linear model. It turned out that when soil OM increased by 1.0%, EK removal ratio (%) of Hg would decrease by 2.63%.









Similar content being viewed by others
References
Acar YB, Alshawabkeh AN (1993) Principles of electrokinetic remediation. Environ Sci Technol 27(13):2638–2647
Cao XD, Chen Y, Wang XR, Deng XH (2001) Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 44:655–661
Cox CD, Shoesmith MA, Ghosh MM (1996) Electrokinetic remediation of mercury-contaminated soils using iodine/iodide lixiviant. Environ Sci Technol 30:1933–1938
de Oliveira LC, Serudo RL et al (2007) Distribution of mercury in different soils of Amazonia’s mid-Negro River basin: influence of organic matter on Mercury’s biogeochemical cycle. Quimica Nova 30(2):274–280
Horvat M, Nolde N, Fajon V et al (2003) Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci Tot Environ 304:231–256
Hylander LD, Meili M (2003) 500 years of mercury production: global annual inventory by region until 2000 and associated emissions. Sci Total Environ 304(1–3):13–27
Ji XL, Hu WX, Cheng JP et al (2006) Oxidative stress on domestic ducks (Shaoxing duck) chronically exposed in a Mercury–Selenium coexisting mining area in China. Ecotoxicol Environ Saf 64(2):171–177
Lucchini R, Calza S, Camerino D et al (2003) Application of a latent variable model for a multicenter study on early effects due to mercury exposure. Neurotoxicology 24:605–616
Martinez-Cortizas A, Pontevedra-Pombal X, Garcia-Rodeja E et al (1999) Mercury in a Spanish Peat Bog: archive of climate change and atmospheric metal deposition. Science 284(7):939–942
Mirlean N, Andrus VE, Baisch P (2003) Mercury pollution sources in sediments of Patos Lagoon Estuary, southern Brazil. Mar Pollut Bull 46:331–334
Ottosen LM, Hansen HK, Laursen S, Villumsen A (1997) Electodialytic remediation of soil polluted from wood preservation industry. Environ Sci Technol 31:1711–1715
Ottosen LM, Pedersen AJ, Ribeiro AB, Hansen HK (2005) Case study on the strategy and application of enhancement solutions to improve remediation of soils contaminated with Cu, Pb and Zn by means of electrodialysis. Gen Geo 77:317–329
Pierzynski GM, Sims JT, Vance GF (2000) Soils and environmental quality, 2nd edn edn. CRC Press, Boca Raton, p 383
Qu LY (2002) The pollution and prevention of mercury in Guizhou province. J Guizhou Normal Univ (Nat Sci) 20:56–59
Reddy KR, Chinthamreddy S (2003) Sequentially enhanced electrokinetic remediation of heavy metals in low buffering clayey soils. J Environ Eng 130(4):442–455
Reddy KR, Chaparro C, Saichek RE (2003) Iodide-enhanced electrokinetic remediation of mercury-contaminated soils. J Environ Eng 129(12):1137–1148
Reynolds WD, Elrick DE (2002) Constant head well permeameter (vadose zone). In: Dane JH, Topp GC (eds) Methods of soil analysis. Part 4. Physical methods. SSSA Book Series 5, Madison, pp 844–858
Rhoades JD (1982) CEC. In: Page AL (ed) Methods of soil analysis, Part 2, Chemical and microbiological properties, 2nd edn edn. ASA, Madison, pp 149–157
Shen ZM, Chen XJ, Jia JP et al (2007) Comparison of electrokinetic soil remediation methods using one fixed anode and approaching anodes. Environ Pollut 150(2):193–199
Shrestha R, Fischer R, Rahner D (2003) Behavior of cadmium, lead and zinc at the sedimentewater interface by electrochemically initiated processes. Colloids Surf A Physicochem Eng Asp 222:261–271
Skyllberg U, Xia K et al (2000) Binding of mercury(II) to reduced sulfur in soil organic matter along upland-peat soil transects. J Environ Qual 29(3):855–865
State Environmental Protection Administration of China (SEPAC) (1995) Environment quality standard for soils, GB15618–1995. Standards Press of China, Beijing, China
Suer P, Lifvergren T (2003) Mercury-contaminated soil remediation by iodide and electroreclamation. J Environ Eng 129(5):441–446
Tan H, He J, He T (1997) Dry and wet deposition of elemental mercury by moss bag near a mercury mine. Environ Sci 18:71–72
USEPA Method 3052 (1996) Microwave assisted acid digestion of siliceous and organically based matrices
Acknowledgments
The authors acknowledge the financial support of the National Natural Science Foundation of China (NO. 28467001 and 20377028) for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, Z., Zhang, J., Qu, L. et al. A modified EK method with an I−/I2 lixiviant assisted and approaching cathodes to remedy mercury contaminated field soils. Environ Geol 57, 1399–1407 (2009). https://doi.org/10.1007/s00254-008-1418-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-008-1418-6