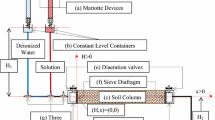
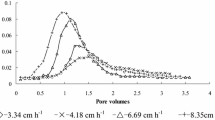
Abstract
There is increasing concern about soil enrichment with K+ and subsequent potential losses following long-term application of poor quality water to agricultural land. Different models are increasingly being used for predicting or analyzing water flow and chemical transport in soils and groundwater. The convective–dispersive equation (CDE) and the convective log-normal transfer function (CLT) models were fitted to the potassium (K+) leaching data. The CDE and CLT models produced equivalent goodness of fit. Simulated breakthrough curves for a range of CaCl2 concentration based on parameters of 15 mmol l−1 CaCl2 were characterised by an early peak position associated with higher K+ concentration as the CaCl2 concentration used in leaching experiments decreased. In another method, the parameters estimated from 15 mmol l−1 CaCl2 solution were used for all other CaCl2 concentrations, and the best value of retardation factor (R) was optimised for each data set. A better prediction was found. With decreasing CaCl2 concentration the value of R is required to be more than that measured (except for 10 mmol l−1 CaCl2), if the estimated parameters of 15 mmol l−1 CaCl2 are used. The two models suffer from the fact that they need to be calibrated against a data set, and some of their parameters are not measurable and cannot be determined independently.







Similar content being viewed by others
References
Beltran JM (1999) Irrigation with saline water: benefits and environmental impact. Agric Water Manage 40:183–194
Boesch DF, Brinsfield RB, Magnien RE (2001) Chesapeake Bay eutrophication: scientific understanding, ecosystem restoration, and challenges for agriculture. J Environ Qual 30(2):303–320
Di HJ, Cameron KC (2004) Effects of the nitrification inhibitor dicyandiamide on potassium, magnesium and calcium leaching in grazed grassland. Soil Use Manage 20:2–7
Elrick DE, Kachanoski RG, Pringle EA, Ward AL (1992) Parameter estimates of field solute transport models based on time domain reflectometry measurements. Soil Sci Soc Am J 56:1663–1666
Gaber HM, Inskeep WP, Comfort SD, Wraith JM (1995) Nonequilibrium transport of atrazine through intact soil cores. Soil Sci Soc Am J 56:60–67
Griffioen J (2001) Potassium adsorption ratios as an indicator for the fate of agricultural potassium in groundwater. J Hydrol 254:244–254
Heng LK , White RE (1996) A simple analytical transfer function approach to modelling the leaching of reactive solutes through field soil. Eur J Soil Sci 47:33–42
Jalali M (2005) Major ion chemistry in the Bahar area, Hamadan, western Iran. Environ Geol 47:763–772
Jalali M (2007) Salinization of groundwater in arid and semi arid zones: an example from Tajarak, western Iran. Environ Geol 52:1133–1149
Jalali M, Rowell DL (2003) The role of calcite and gypsum in the leaching of potassium in a sandy soil. Exp Agric 39:379–394
Jardine PM, Wilson GV, Luxmoore RJ (1988) Modelling the transport of inorganic ions through undisturbed soil columns from two contrasting watersheds. Soil Sci Soc Am J 52:1252–1259
Jardine PM, Jacobs GK, Wilson GV (1993) Unsaturated transport processes in undisturbed heterogeneous porous media: I. Inorganic contaminants. Soil Sci Soc Am J 57:945–953
Jury WA (1982) Simulation of solute transport using a transfer function model. Water Resour Res 18:363–368
Jury WA, Roth K (1990) Transfer functions and solute movement through soil: theory and applications. Birkhauser, Basel
Khan AUH, Jury WA (1990) A laboratory test of the dispersion scale effect. J Contam Hydrol 5:119–132
Kolahchi Z, Jalali M (2007) Effect of water quality on the leaching of potassium from sandy soil. J Arid Environ 68:624–239
Lapidus L, Amundson NR (1952) Mathematics of adsorption in beds. VI. The effect of longitudinal diffusion in ion exchange and chromatographic columns. J Phys Chem 56:984–988
Maguire RO, Sims JT (2002) Measuring agronomic and environmental soil phosphorus saturation and predicting phosphorus leaching with Mehlich 3. Soil Sci Soc Am J 66(6):2033–2039
Parker JC, van Genuchten MTh (1984) Determining transport parameters from laboratory and field tracer experiments. Bulletin 84-3. Virginia Agriculture Experiment Station, Blackburg
Rao PSC, Davidson JM, Jessup RE, Selim HM (1979) Evaluation of conceptual models for describing nonequilibrium adsorption–desorption of pesticides during steady-flow in soils. Soil Sci Soc Am J 43:22–28
Ross SM (1994) Retention, transformation and mobility of toxic metals in soil. In: Ross SM (ed). Toxic metals in soil–plant systems. Wiley, Chichester, pp 63–152
Simmonds LP, Nortcliff S (1998) Small scale variability in the flow of water and solutes, and implications for lysimeter studies of solute leaching. Nutr Cycl Agroecosys 50(1–3):67–75
Valocchi AJ (1985) Validity of the local equilibrium assumption for modelling sorbing solute transport through homogeneous soils. Water Resour Res 21:808–820
van Genuchten MTH, Wierenga PJ (1976) Mass transfer studies in sorbing porous media. I. Analytical solutions Soil Sci Soc Am J 40:473–480
Wagenet RJ (1983) Principles of salt movement in soil. In: Nelson DW, Elric DE, Tanji KK (eds) Chemical mobility and reactivity in soil systems. Soil Sci Soc Am WI Publication No. 11, Madison, pp 123–140
WHO (1993) Guidelines for drinking water quality, 1. Recommendations, 2nd edn. World Health Organisation, Geneva
Zhang R (1995) Prediction of solute transport using transfer function model and the convection–dispersion equation. Soil Sci 160:18–27
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jalali, M., Rowell, D.L. Prediction leaching of potassium using the convective–dispersive and the convective log-normal transfer function models. Environ Geol 55, 863–874 (2008). https://doi.org/10.1007/s00254-007-1038-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-1038-6