Abstract
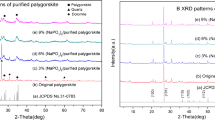
In this paper, Shuwaymiyah palygorskite in the Sultanate of Oman has been characterized mineralogically by X-ray diffraction (XRD) and electron microscopy, chemically by oxide compositions, structural formulae, and cation exchange capacity (CEC), and physically by specific surface area and adsorption isotherms. Batch adsorption studies were performed to evaluate the adsorption performance of methylene blue (MB) basic dye on the local clay mineral. The quantitative XRD analysis indicates that the purity of some selected samples of palygorskite clay is very high (about 70% of the clay minerals are palygorskite and 30% kaolinite). The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images clearly support this conclusion. The adsorption equilibrium revealed that Shuwaymiyah palygorskite clay can uptake up to 51 mg of MB per 1 g mass of clay. MB adsorption is best fitted by Langmuir isotherm, and a pseudo-second-order kinetic model can be efficiently used to predict the kinetic of adsorption of MB by the palygorskite. The results obtained from these laboratory-scale adsorption tests indicate the promising adsorption capability of the Omani palygorskite.








Similar content being viewed by others
References
Al-Rawas AA, Sutherland H, Hago AW, Yousif AA, Al-Shihi M, Al-Shihi B (2001) A comparative quantitative study of an Omani soil using X-ray diffraction technique. J Geotechnical Geol Eng 19:69–84
Bailey W (1980) Structure of layer silicate. In: Brindley GW, Brown G (eds) Crystal structures of clay minerals and their X-ray identification. Monograph 5. Mineralogical Society, London. pp 1–123
Bajjali W (1996) Groundwater contamination by hydrocarbon in Nizwa, Zukait, Jaalan Bani Bu Hasan, and Barka areas. Technical report, Ministry of Water Resources, Oman
Broderick GP, Daniel DE (1990) Stabilizing compacted clay against chemical attack. J Geotechnical Eng 116(10):1549–1567
Chahi A, Duplay J, Lucas J (1993) Analysis of palygorskite and associated clays from the Jbel Rhassoul (Morocco): chemical characteristics and origin of formation. Clays Clay Miner 41(4):401–411
Chang SH, Ryan ME, Gupta RK (1991) Competitive adsorption of water-soluble polymers on attapulgite clay. J Appl Polym Sci 43(7):1293–1299
El-Zawahry A, Shahalam AM, Taha R, Al-Busaidi T (2001) An assessment of solid waste and landfills in Muscat area, Oman. Sci Technol SQU 6:1–11
Galan E (1996) Properties and applications of palygorskite–sepiolite clays. Clay Miner 31:443–453
Galan E, Carretero I (1999) A new approach to compositional limits for sepiolite and palygorskite. Clays Clay Miner 47:399–409
Garcia-Romero E, Sauarez Barrios M, Bustillo Revuelta MA (2004) Characteristics of a Mg-palygorskite in Miocene rocks, Madrid basin (Spain). Clays Clay Miner 52(4):484–494
Gurbe WE (1992) Slurry trench cut-off walls for environmental pollution control. In: ASTM Special Technical Publication, Symposium on slurry walls: design, construction, and quality control, Atlantic City, NJ, USA. Sponsor: ASTM, pp 67–77
Hang PT, Brindley GW (1970) Methylene blue adsorption by clay minerals: determination of surface areas and cation exchange capacities (clay-organic X VIII). Clays Clay Miner 18:203–212
Haydn MH (1991) Some applications of selected clay minerals. In: Applied clay science, 24th Annual Meeting of the Clay Minerals Society, 18–22 October 1987, NM, USA, pp 379–395
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742
Minerals Division (2002) Shuwaymiya and Tawi Attair Attapulgite. Technical report, Ministry of Commerce and Economy, Oman
Murray HH (2000) Traditional and new applications for kaolin, smectite, and palygorskite: a general overview. Appl Clay Sci 17:207–221
Newman AC, Brown G (1987) Palygorskite and sepiolite. In: Newman AC (ed) Chemistry of clays and clay minerals. Monograph 6, Mineralogical Society, London
Pamukcu S, Hijazi H (1992) Improvement of fuel oil contaminated soils by additives. In: Geotechnical Special Publication. In: Proceedings of the 1992 ASCE Specialty Conference on Grouting, Soil improvement and Geosynthetics, 25–28 February, 1992, New Orleans, LA, USA, pp 1285–1297
Paquet H, Duplay J, Valleron M, Millot G (1987) Octahedral compositions of individual particles in smectite-palygorskite and smectite-sepiolite assemblages. In: Proceedings of the 8th International Clay Conference, Denver. The Clay Minerals Society, Bloomington, pp 73–77
Pillon LZ (2001) Effect of attapulgite clay on the composition of jet fuels. Petroleum Sci Technol 19(7–8):875–884
Rohe RV, Olson CG (1979) Estimate of clay–mineral content: additions of proportions of soil clay to constant standard. Clays Clay Miner 27(5):322–326
Rytow R, Serban C, Nir S, Margulies L (1991) Use of methylene blue and crystal violet for determination of exchangeable cations in montmorillonite. Clays Clay Miner 39(5):551–555
Scheu M (1993) Assessment study on municipal solid waste management in Muscat. Technical report. Kalwasswerp Engineering, Wetter
Vinod VP, Anirudhan TS (2003) Adsorption behaviour of basic dyes on the humic acid immobilized pillared clay. Water Air Soil Pollut 150:193–217
Wang L, Huang Z, Sun X, Liu X, Jin J (1998) Treating dyeing wastewater with modified absorbent of attapulgite. J Nanjing Univ Sci Technol 22(3):240–243
Wu F, Tseng R, Juang R (2001) Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res 35(3):613–618
Zunan Q, Yi Z, Yuqiao F (1995) Removal of oil from concentrated wastewater by attapulgite and coagulant. Water Qual Res J Can 30(1):89–99
Acknowledgement
This study has been supported by Sultan Qaboos University Internal Grant IG/ENG/CAED/04/02. The support of the Directorate of Mineral Exploration in the Ministry of Commerce and Industry (Salim Al-Busaidi and Khalid Al-Toobi) is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Futaisi, A., Jamrah, A., Al-Rawas, A. et al. Adsorption capacity and mineralogical and physico-chemical characteristics of Shuwaymiyah palygorskite (Oman). Environ Geol 51, 1317–1327 (2007). https://doi.org/10.1007/s00254-006-0430-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0430-y