Abstract
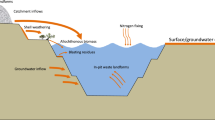
Hydrogeochemical processes controlling surface water chemistry were examined in five small (<1.5 km2) forested catchments that have contrasting bedrock lithologies of granite, and conglomerate, and are distributed in the southeast of Seto district, central Japan. Watersheds developed on these two bedrocks differ in their ability to neutralize atmospheric acid (pH ~4.5) deposition. The study was conducted to (1) characterize the hydrogeochemical processes controlling surface water chemistry, and (2) to elucidate acidification of spring and stream waters using data from three sampling campaigns conducted from August to October 2000. Stream and spring water solutes fall into two general groups according to concentration: alkaline, relatively high pH (5.2–7.7) and high cation concentrations (HCO3 −, Cl−, base cations), and dilute, low pH (4.2–5.5) waters. Concentrations of trace metals (Al, Ba, Sr) showed a strong negative correlation with pH, suggesting the mobility of these metals in the dissolved load of catchments underlain by Tokai conglomerate. The strontium isotope ratio (87Sr/86Sr) of rock and soil, plant, precipitation, and surface water samples was used to identify different reservoirs within the ecosystem. Low Si concentrations in stream and spring waters from the conglomerate area, with a relatively high pool of SiO2, >90 (wt%), suggest slow chemical weathering. The dissolved solute concentrations are generally of similar magnitude in stream waters within the catchments of similar bedrock lithology. The high inverse correlation (r 2=0.72) between pH and SO4 – concentrations and the high positive correlation (r 2 =0.90) between Ba and SO4 – concentrations in waters draining Tokai conglomerate suggest that barite (BaSO4) is being dissolved in an acidic environment. The three catchments were identified as being sensitive to acidic deposition because the bedrock conglomerate provided little capacity to buffer acidic inputs. The soils from the granite area have a high cation-exchange capacity (CEC an average of 868 µmol/kg), and are nearly ten times greater than the soils from the conglomerate area. Because ion exchange, besides weathering, is the main source that counteracts soil acidification, the sensitivity to further acidification may increase.









Similar content being viewed by others
References
Bache BW (1986) Aluminium mobilization in soils and waters. J Geol Soc 143:699–706
Bailey W, Hornbeck JW, Driscoll CT, Gaudette HE (1996) Calcium inputs and transport in a base-poor forest ecosystem as interpreted by Sr isotopes. Water Resour Res 32:707–719
Bain DC, Roe MJ, Duthie DML, Thomson CM (2001) The influence of mineralogy on weathering rates and processes in an acid-sensitive granitic catchment. Appl Geochem 16:931–937
Barrett CF, Atkins DHF, Cape JN, Fowler D, Irwin JG, Kallend AS, Martin A, Pitman JI, Scriven RA, Tuck AF (1983) Acid deposition in the United Kingdom. Warren Spring Laboratory, Stevenage
Bergaya F, Vayer M (1997) CEC of clays: measurement by adsorption of a copper ethylenediamine complex. Appl Clay Sci 12:275–280
Bigorre F, Tessier D, Pedro G (2000) Significance of CEC and surface area of soils. How clay and organic matter contribute to water retention properties. Earth Planet Sci 330:245–250
Capo RC, Stewart BW, Chadwick OA (1998) Strontium isotopes as tracers of ecosystem processes: theory and method. Geoderma 82:197–225
Craw D (2000) Water–rock interaction and acid neutralization in a large schist debris dam, Otago, New Zealand. Chem Geol 171:17–32
Dovland H (1993) EMEP—the European monitoring and evaluation programme. Proceedings of the Expert Meeting on Acid Precipitation Network in East Asia, 26–28 October 1993. Toyama, Japan, pp 34–35
Drever JI (1997a) Weathering processes. In: Saether OM, de Caritat P (eds) Geochemical processes, weathering and groundwater recharge in catchments. Balkema, Rotterdam, pp 3–19
Drever JI (1997b)Catchment mass balance. In: Saether OM, de Caritat P (eds) Geochemical processes, weathering and groundwater recharge in catchments. Balkema, Rotterdam, pp 241–261
Drever JI, Stillings LL (1997) The role of organic acids in mineral weathering. Colloid Surfaces A 120:167–181
Edmunds WM, Kinniburgh DG (1986) The susceptibility of UK groundwaters to acidic deposition. J Geol Soc Lond 143:707–720
Feger KH, Brahmer G, Zottl HW (1990) Element budgets of two contrasting catchments in the black forest (Federal Republic of Germany). J Hydrol 116:85–99
Frei M, Bielert U, Heinrich H (2000) Effects of pH, alkalinity and bedrock chemistry on metal concentrations of springs in an acidified catchment (Ecker Dam, Harz Mountains, FRG). Chem Geol 170:221–242
Fritz SJ (1988) A comparative study of gabbro and granite weathering. Chem Geol 68:275–290
Herut B, Starinsky A, Katz A, Rosenfeld D (2000) Relationship between the acidity and chemical composition of rainwater and climatological conditions along a transition zone between large deserts and Mediterranean climate, Israel. Atmos Environ 34:1281–1292
Ishikawa Y, Yoshimura K, Mori A, Hara H (1998) High sulfate and nitrate concentrations in precipitation at Nagasaki impacted by long-distant and local sources. Atmos Environ 32:2939–2945
Jenkins A, Whitehead PG, Musgrove TJ, Cosby BJ (1990) A regional model of acidification in Wales. J Hydrol 116:403–416
Johnson NM (1984) Acid rain neutralization by geological materials. In: Bricker OP (ed) Geological aspects of acid deposition. Acid precipitation series vol 7. Butterworth, Boston, pp 37–53
Kamoshita Y (1958) Soils in Japan. National Institute of Agricultural Sciences, Nishigahara, Kita-Ku, Tokyo, pp 5, 9
Likens GE, Wright RF, Galloway JN, Butler TJ (1979) Acid rain. Sci Am 241:43–50
Ludwig B, Khanna PK, Anurugsa B, Folster H (2001) Assessment of cation and anion exchange and pH buffering in an Amazonian Ultisol. Geoderma 102:27–40
Moriyama A (2000) Wetland ecology and geology of the Kaisyo Forest in southeastern part of Seto City, central Japan: critical comments of environmental assessment for word EXPO 2005 (in Japanese). Jpn J Conserv Ecol 5:7–41
Na Choon-ki, Nakano T, Tazawa K, Sakagawa M, Ito T (1995) A systematic and practical method of liquid chromatography for the determination of Sr and Nd isotopic ratios and REE concentrations in geological samples. Chem Geol 123:225–237
Nakagawa Y, Iwatsubo G (2000) Water chemistry in a number of mountainous streams of east Asia. J Hydrol 240:118–130
Nakayama K, Yoshikawa S (1997) Depositional processes of primary to reworked volcaniclastics on an alluvial plain; an example from the Lower Pliocene Ohta tephra bed of the Tokai Group, central Japan. Sediment Geol 107:211–229
Nakayama K, Yoshikawa S, Ito T (1995) Magnetostratigraphy of the Late Cenozoic Tokai Group in central Japan and its sedimentologic implications. J SE Asian Earth Sci 12:95–104
Neal C, Rosier PTW (1990) Chemical studies of chloride and stable oxygen isotopes in two conifer afforested and Moorland sites in the British uplands. J Hydrol 115:269–283
Negrel P, Deschamps P (1996) Natural and anthropogenic budgets of a small watershed in the Massif Central (France). Chemical and strontium isotopic characterization in water and sediments. Aquat Geochem 2:1–27
Nordstrom DK (1982) The effect of sulfate on aluminum concentrations in natural waters: some stability relations in the system Al2O3–SO3–H2O at 298 K. Geochim Cosmochim 46:681–692
Ohrui K, Mitchell MJ (1998) Stream water chemistry in Japanese forested watersheds and its variability on a small regional scale. Water Resour Res 6:1553–1561
Patterson C (1974) Lead in seawater. Science 183:553–558
Petelet E, Luck JM, Ben Othman D, Negrel P, Aquilina L (1998) Geochemistry and water dynamics of a medium-sized watershed: the Herault, southern France, 1. Organisation of the different water reservoirs as constrained by Sr isotopes, major, and trace elements. Chem Geol 150:63–83
Reuss JO, Johnson DW (1986) Acid deposition and the acidification of soils and waters. Springer, Berlin Heidelberg New York
Reuss JO, Cosby BJ, Wright RF (1987) Chemical processes governing soil and water acidification. Nature 329:27–32
Robson A, Neal C (1990) Hydrograph separation using chemical techniques: an application to catchments in Mid-Wales. J Hydrol 116:345–363
Rosseland BO, Eldhuset TD, Staurnes M (1990) Environmental effects of aluminum. Environ Geochem Health 12:17–27
Seip HM, Andersen S, Henriksen A (1990) Geochemical control of aluminium concentrations in acidified surface waters. J Hydrol 116:299–305
Soulsby C, Turnbull D, Hirst D, Langan SJ, Owen R (1997) Reversibility of stream acidification in the Cairngorm region of Scotland. J Hydrol 195:291–311
Stauffer RE, Wittchen BD (1991) Effects of silicate weathering on water chemistry in forested, upland, felsic terrane of the USA. Geochim Cosmochim Acta 55:3253–3271
Steinberg CEW, Wright RF (eds) (1994) Acidification of freshwater ecosystems. Wiley, Chichester
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley, Chichester
Tamaki M, Kato T, Sekiguchi K, Kitamura M, Taguchi K, Ohara M, Mori A, Wakamatsu S, Murano K, Okita T, Yamanaka Y, Hara H (1991) Acid precipitation over Japan. Nippon Kagakukaishi pp 667–674
Van Breeman N, Driscroll TC, Mulder J (1984) Acidic deposition and internal proton sources in acidification of soils and waters. Nature 307:599–604
Wang TJ, Jin LS, Li ZK, Lam KS (2000) A modeling study on acid rain and recommended emission control strategies in China, Atmos Environ 34:4467–4477
Wright RF (1983) Acidification of fresh waters in Europe. Water Qual Bull 8:137–142
Yokoo Y (2000) Geochemical study of desert sand and loess in China: implications for the provenance and formation of Japanese soil. PhD Thesis, University of Tsukuba, Japan
Acknowledgements
The author would like to thank the Ministry of Education, Japan, for providing a scholarship. I am grateful to Prof. Y. Ogawa for his guidance in the literature survey. Also thanks to Mr. and Mrs. A. Kikuchi for their support during field sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sultan, K. Geochemical and Sr-isotopic characteristics of stream and spring waters from small-forested catchments at Seto, central Japan. Env Geol 44, 308–324 (2003). https://doi.org/10.1007/s00254-003-0763-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-003-0763-8