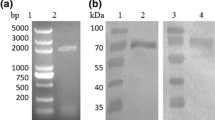
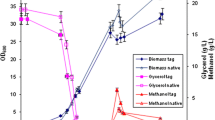
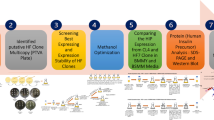
Abstract
Insulin-like growth factor-1 (IGF-1) is a pleiotropic protein hormone and has become an attractive therapeutic target because of its multiple roles in various physiological processes, including growth, development, and metabolism. However, its production is hindered by low heterogenous protein expression levels in various expression systems and hard to meet the needs of clinical and scientific research. Here, we report that human IGF-1 and its analog Long R3 IGF-1 (LR3 IGF-1) are recombinant expressed and produced in the Pichia pastoris (P. pastoris) expression system through being fused with highly expressed xylanase XynCDBFV. Furthermore, purified IGF-1 and LR3 IGF-1 display excellent bioactivity of cell proliferation compared to the standard IGF-1. Moreover, higher heterologous expression levels of the fusion proteins XynCDBFV-IGF-1 and XynCDBFV-LR3 IGF-1 are achieved by fermentation in a 15-L bioreactor, reaching up to about 0.5 g/L XynCDBFV-IGF-1 and 1 g/L XynCDBFV-TEV-LR3 IGF-1. Taken together, high recombinant expression of bioactive IGF-1 and LR3 IGF-1 is acquired with the assistance of xylanase as a fusion partner in P. pastoris, which could be used for both clinical and scientific applications.
Key points
• Human IGF-1 and LR3 IGF-1 are produced in the P. pastoris expression system.
• Purified IGF-1 and LR3 IGF-1 show bioactivity comparable to the standard IGF-1.
• High heterologous expression of IGF-1 and LR3 IGF-1 is achieved by fermentation in a bioreactor.




Similar content being viewed by others
Data availability
All materials are available by the corresponding author upon reasonable request.
References
Aboutalebi F, Lachinani L, Khazaei Y, Forouzanfar M, Nasr-Esfahani MH, Ghaedi K, Dormiani K (2018) An efficient method for bacterial production and activity assessment of recombinant human insulin like growth factor 1. Mol Biol Rep 45(6):1957–1966. https://doi.org/10.1007/s11033-018-4348-8
Araujo MS, Guastali MD, Paulini F, Silva AN, Tsunemi MH, Fontes PK, Castilho ACS, Landim-Alvarenga FC (2020) Molecular and cellular effects of insulin-like growth factor-1 and LongR3-IGF-1 on in vitro maturation of bovine oocytes: comparative study. Growth Horm IGF Res 55:101357. https://doi.org/10.1016/j.ghir.2020.101357
Bach LA (2004) The insulin-like growth factor system: towards clinical applications. Clin Biochem Rev 25(3):155–164
Baeshen MN, Bouback TA, Alzubaidi MA, Bora RS, Alotaibi MA, Alabbas OT, Alshahrani SM, Aljohani AA, Munshi RA, Al-Hejin A, Ahmed MM, Redwan EM, Ramadan HA, Saini KS, Baeshen NA (2016) Expression and purification of C-peptide containing insulin using Pichia pastoris expression system. Biomed Res Int 2016:3423685. https://doi.org/10.1155/2016/3423685
Barner C, Petersson M, Eden Engstrom B, Hoybye C (2012) Effects on insulin sensitivity and body composition of combination therapy with GH and IGF1 in GH deficient adults with type 2 diabetes. Eur J Endocrinol 167(5):697–703. https://doi.org/10.1530/EJE-12-0484
Bourbonnais Y, Bolin D, Shields D (1988) Secretion of somatostatin by Saccharomyces cerevisiae. Correct proteolytic processing of pro-alpha-factor-somatostatin hybrids requires the products of the KEX2 and STE13 genes. J Biol Chem 263(30):15342–15347
Brierley RA (1998) Secretion of recombinant human insulin-like growth factor I (IGF-I). Methods Mol Biol 103:149–177. https://doi.org/10.1385/0-89603-421-6:149
Chen X, Zaro JL, Shen WC (2013) Fusion protein linkers: property, design and functionality. Adv Drug Deliv Rev 65(10):1357–1369. https://doi.org/10.1016/j.addr.2012.09.039
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29(1):3–23. https://doi.org/10.1016/j.femsre.2004.06.005
Cui L, Huang H, Zhang H, Wang X, Qin X, Tu T, Zhang J, Su X, Yu H, Bai Y, Luo H, Yao B, Wang Y (2022) Recombinant expression of hen egg white lysozyme with the assistance of xylanase fusion partner in Pichia pastoris. Bioengineered 13(5):13860–13871. https://doi.org/10.1080/21655979.2022.2084496
Emamipour N, Vossoughi M, Mahboudi F, Golkar M, Fard-Esfahani P (2019) Soluble expression of IGF1 fused to DsbA in SHuffle T7 strain: optimization of expression and purification by Box-Behnken design. Appl Microbiol Biotechnol 103(8):3393–3406. https://doi.org/10.1007/s00253-019-09719-w
Fang W, Gao H, Cao Y, Shan A (2014) Cloning and expression of a xylanase xynB from Aspergillus niger IA-001 in Pichia pastoris. J Basic Microbiol 54(Suppl 1):S190–S199. https://doi.org/10.1002/jobm.201300078
Francis GL, Ross M, Ballard FJ, Milner SJ, Senn C, McNeil KA, Wallace JC, King R, Wells JR (1992) Novel recombinant fusion protein analogues of insulin-like growth factor (IGF)-I indicate the relative importance of IGF-binding protein and receptor binding for enhanced biological potency. J Mol Endocrinol 8(3):213–223. https://doi.org/10.1677/jme.0.0080213
Frysak Z, Schovanek J, Iacobone M, Karasek D (2015) Insulin-like growth factors in a clinical setting: review of IGF-I. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 159(3):347–351. https://doi.org/10.5507/bp.2015.041
Fuqua JS, Derr M, Rosenfeld RG, Hwa V (2012) Identification of a novel heterozygous IGF1 splicing mutation in a large kindred with familial short stature. Horm Res Paediatr 78(1):59–66. https://doi.org/10.1159/000337249
Goodrick JC, Xu M, Finnegan R, Schilling BM, Schiavi S, Hoppe H, Wan NC (2001) High-level expression and stabilization of recombinant human chitinase produced in a continuous constitutive Pichia pastoris expression system. Biotechnol Bioeng 74(6):492–497. https://doi.org/10.1002/bit.1140
Hakuno F, Takahashi SI (2018) IGF1 receptor signaling pathways. J Mol Endocrinol 61(1):T69–T86. https://doi.org/10.1530/JME-17-0311
Huang K, Chu Y, Qin X, Zhang J, Bai Y, Wang Y, Luo H, Huang H, Su X (2021) Recombinant production of two xylanase-somatostatin fusion proteins retaining somatostatin immunogenicity and xylanase activity in Pichia pastoris. Appl Microbiol Biotechnol 105(10):4167–4175. https://doi.org/10.1007/s00253-021-11298-8
Julius D, Blair L, Brake A, Sprague G, Thorner J (1983) Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell 32(3):839–852. https://doi.org/10.1016/0092-8674(83)90070-3
Karbalaei M, Rezaee SA, Farsiani H (2020) Pichia pastoris: a highly successful expression system for optimal synthesis of heterologous proteins. J Cell Physiol 235(9):5867–5881. https://doi.org/10.1002/jcp.29583
Khamsi F, Armstrong DT (1997) Interactions between follicle-stimulating hormone and growth factors in regulation of deoxyribonucleic acid synthesis in bovine granulosa cells. Biol Reprod 57(3):684–688. https://doi.org/10.1095/biolreprod57.3.684
Kim SO, Lee YI (1996) High-level expression and simple purification of recombinant human insulin-like growth factor I. J Biotechnol 48(1–2):97–105. https://doi.org/10.1016/0168-1656(96)01402-2
Miki H, Takagi M (2015) Design of serum-free medium for suspension culture of CHO cells on the basis of general commercial media. Cytotechnology 67(4):689–697. https://doi.org/10.1007/s10616-014-9778-0
Panahi M, Alli Z, Cheng X, Belbaraka L, Belgoudi J, Sardana R, Phipps J, Altosaar I (2004) Recombinant protein expression plasmids optimized for industrial E. coli fermentation and plant systems produce biologically active human insulin-like growth factor-1 in transgenic rice and tobacco plants. Transgenic Res 13(3):245–259. https://doi.org/10.1023/b:trag.0000034619.21613.d0
Puche JE, Castilla-Cortazar I (2012) Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J Transl Med 10:224. https://doi.org/10.1186/1479-5876-10-224
Romand S, Jostock T, Fornaro M, Schmidt J, Ritter A, Wilms B, Laux H (2016) Improving expression of recombinant human IGF-1 using IGF-1R knockout CHO cell lines. Biotechnol Bioeng 113(5):1094–1101. https://doi.org/10.1002/bit.25877
Rosenbloom AL (2009) Mecasermin (recombinant human insulin-like growth factor I). Adv Ther 26(1):40–54. https://doi.org/10.1007/s12325-008-0136-5
TurkanogluOzcelik A, Yilmaz S, Inan M (2019) Pichia pastoris promoters. Methods Mol Biol 1923:1997–2112. https://doi.org/10.1007/978-1-4939-9024-5_3
Vai M, Brambilla L, Orlandi I, Rota N, Ranzi BM, Alberghina L, Porro D (2000) Improved secretion of native human insulin-like growth factor 1 from gas1 mutant Saccharomyces cerevisiae cells. Appl Environ Microbiol 66(12):5477–5479. https://doi.org/10.1128/AEM.66.12.5477-5479.2000
Wan A, Xu D, Liu K, Peng L, Cai Y, Chen Y, He Y, Yang J, Jin J, Li H (2017) Efficient expression of stable recombinant human insulin-like growth factor-1 fusion with human serum albumin in Chinese hamster ovary cells. Prep Biochem Biotechnol 47(7):678–686. https://doi.org/10.1080/10826068.2017.1303612
Funding
The authors are grateful to the National Key Research and Development Program of China (2022YFD1300701, 2019YFE0115000) and the China Agriculture Research System of MOF and MARA (CARS-41).
Author information
Authors and Affiliations
Contributions
H.Z. designed and supervised the research, analyzed the data, and wrote the manuscript. Z.L. and N.L. performed the experiments, analyzed the data, and revised the manuscript. H.H. and Y.W. analyzed the data and revised the manuscript. T.T., X.Q., X.W., J.Z., X.S., J.T., and Y.B. analyzed the data. H.L. and B.Y. contributed new reagents or analytical tools. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Human and animal rights
This work does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Z., Liu, N., Huang, H. et al. Recombinant expression of IGF-1 and LR3 IGF-1 fused with xylanase in Pichia pastoris. Appl Microbiol Biotechnol 107, 4543–4551 (2023). https://doi.org/10.1007/s00253-023-12606-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12606-0