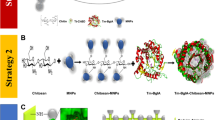
Abstract
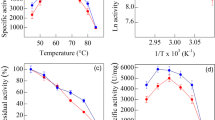
A novel chitinase gene of 888 bp from Streptomyces bacillaris was cloned and expressed in Escherichia coli BL21. The purified recombinant enzyme (SbChiAJ103) was identified as the first microbial-derived family 19 endochitinase that showed exochitinase activity. SbChiAJ103 exhibited the substrate preference for N-acetylchitooligosaccharides with even degrees of polymerization and the capability to specifically hydrolyze colloidal chitin into (GlcNAc)2. Mono-methyl adipate was employed as a novel linker for the efficient covalent immobilization of chitinase on magnetic nanoparticles (MNPs). The immobilized SbChiAJ103, SbChiAJ103@MNPs, exhibited superior pH tolerance, temperature stability, and storage stability than free SbChiAJ103. Even after incubation at 45 °C for 24 h, SbChiAJ103@MNPs could retain more than 60.0% initial activity. As a result, the enzymatic hydrolysis yield of SbChiAJ103@MNPs increased to 1.58 times that of free SbChiAJ103. Moreover, SbChiAJ103@MNPs could be reused by convenient magnetic separation. After 10 recycles, SbChiAJ103@MNPs could retain almost 80.0% of its initial activity. The immobilization of the novel chitinase SbChiAJ103 paves the way to the efficient and eco-friendly commercial production of (GlcNAc)2.
Key points
• The first microbial GH19 endochitinase with exochitinase activity was reported.
• Mono-methyl adipate was first employed to immobilize chitinase.
• SbChiAJ103@MNPs showed excellent pH stability, thermal stability, and reusability.
Graphical Abstract








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Bai L, Kim J, Son K, Chung C, Shin D, Ku B, Kim D, Park H (2021) Novel bi-modular GH19 chitinase with broad pH stability from a fibrolytic intestinal symbiont of Eiseniafetida, Cellulosimicrobiumfunkei HY-13. Biomolecules 11:1735. https://doi.org/10.3390/biom11111735
Barbosa O, Ortiz C, Berenguer-Murcia A, Torres R, Rodrigues RC, Fernandez-Lafuente R (2014) Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. Rsc Adv 4:1583–1600. https://doi.org/10.1039/c3ra45991h
Bradford MM (1976) A rapid and sensitive method for the quantitation quantities microgram principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37:D233–D238. https://doi.org/10.1093/nar/gkn663
Chen J, Shen C, Liu C (2010) N-Acetylglucosamine: production and applications. Mar Drugs 8:2493–2516. https://doi.org/10.3390/md8092493
Chen W, Jiang X, Yang Q (2020) Glycoside hydrolase family 18 chitinases: the known and the unknown. Biotechnol Adv 43:107553. https://doi.org/10.1016/j.biotechadv.2020.107553
Delfino I, Portaccio M, Ventura BD, Mita DG, Lepore M (2013) Enzyme distribution and secondary structure of sol–gel immobilized glucose oxidase by micro-attenuated total reflection FT-IR spectroscopy. Mater Sci Eng, C 33:304–310. https://doi.org/10.1016/j.msec.2012.08.044
Doan CT, Tran TN, Wang S (2021) Production of thermophilic chitinase by Paenibacillus sp. TKU052 by bioprocessing of chitinous fishery wastes and its application in N-acetyl-d-glucosamine production. Polymers-Basel 13:3048. https://doi.org/10.3390/polym13183048
Ein Ali Afjeh M, Pourahmad R, Akbari-Adergani B, Azin M (2020) Characteristics of glucose oxidase immobilized on magnetic chitosan nanoparticles. Food Sci Tech-Brazil 40:68–75. https://doi.org/10.1590/fst.32618
Fujita K, Shimomura K, Yamamoto K, Yamashita T, Suzuki K (2006) A chitinase structurally related to the glycoside hydrolase family 48 is indispensable for the hormonally induced diapause termination in a beetle. Biochem Bioph Res Co 345(1):502–507. https://doi.org/10.1016/j.bbrc.2006.04.126
Gaber Y, Mekasha S, Vaaje-Kolstad G, Eijsink VGH, Fraaije MW (2016) Characterization of a chitinase from the cellulolytic actinomycete Thermobifidafusca. Biochim et Biophys Acta (BBA) - Proteins Proteomics 1864:1253–1259. https://doi.org/10.1016/j.bbapap.2016.04.010
Gao L, Sun J, Secundo F, Gao X, Xue C, Mao X (2018) Cloning, characterization and substrate degradation mode of a novel chitinase from Streptomyces albolongus ATCC 27414. Food Chem 261:329–336. https://doi.org/10.1016/j.foodchem.2018.04.068
Guo H, Lei B, Yu J, Chen Y, Qian J (2021) Immobilization of lipase by dialdehyde cellulose crosslinked magnetic nanoparticles. Int J Biol Macromol 185:287–296. https://doi.org/10.1016/j.ijbiomac.2021.06.073
Hajji S, Younes I, Ghorbel-Bellaaj O, Hajji R, Rinaudo M, Nasri M, Jellouli K (2014) Structural differences between chitin and chitosan extracted from three different marine sources. Int J Biol Macromol 65:298–306. https://doi.org/10.1016/j.ijbiomac.2014.01.045
Hoell IA, Dalhus B, Heggset EB, Aspmo SI, Eijsink VGH (2006) Crystal structure and enzymatic properties of a bacterial family 19 chitinase reveal differences from plant enzymes. FEBS J 273:4889–4900. https://doi.org/10.1111/j.1742-4658.2006.05487.x
Hosseini SH, Hosseini SA, Zohreh N, Yaghoubi M, Pourjavadi A (2018) Covalent immobilization of cellulase using magnetic poly(ionic liquid) support: improvement of the enzyme activity and stability. J Agr Food Chem 66:789–798. https://doi.org/10.1021/acs.jafc.7b03922
Hoster F, Schmitz JE, Daniel R (2005) Enrichment of chitinolytic microorganisms: isolation and characterization of a chitinase exhibiting antifungal activity against phytopathogenic fungi from a novel Streptomyces strain. Appl Microbiol Biot 66:434–442. https://doi.org/10.1007/s00253-004-1664-9
Jeffrey Yuan AAJB (1999) Multiclustal_ a systematic method for surveying ClustalW alignment parameters. Bioinformatics 15(10):862–863. https://doi.org/10.1093/bioinformatics/15.10.862
Kawase TNUJ, Yokokawa S, Saito A, Fujii T, Nikaidou N, Miyashita K, Watanabe T (2006) Comparison of enzymatic and antifungal properties between family 18 and 19 chitinases from S. coelicolor A3(2). Biosci Biotechnol Biochem 70:988–998. https://doi.org/10.1271/bbb.70.988
Kezuka Y, Ohishi M, Itoh Y, Watanabe J, Mitsutomi M, Watanabe T, Nonaka T (2006) Structural studies of a two-domain chitinase from Streptomyces griseus HUT6037. J Mol Biol 358:472–484. https://doi.org/10.1016/j.jmb.2006.02.013
Kidibule PE, Costa J, Atrei A, Plou FJ, Fernandez-Lobato M, Pogni R (2021) Production and characterization of chitooligosaccharides by the fungal chitinase Chit42 immobilized on magnetic nanoparticles and chitosan beads: selectivity, specificity and improved operational utility. Rsc Adv 11:5529–5536. https://doi.org/10.1039/d0ra10409d
Lee SG, Koh HY, Han SJ, Park H, Na DC, Kim I, Lee HK, Yim JH (2010) Expression of recombinant endochitinase from the Antarctic bacterium, Sanguibacterantarcticus KOPRI 21702 in Pichia pastoris by codon optimization. Protein Expres Purif 71:108–114. https://doi.org/10.1016/j.pep.2010.01.017
Lei L, Liu X, Li Y, Cui Y, Yang Y, Qin G (2011) Study on synthesis of poly(GMA)-grafted Fe3O4/SiOX magnetic nanoparticles using atom transfer radical polymerization and their application for lipase immobilization. Mater Chem Phys 125:866–871. https://doi.org/10.1016/j.matchemphys.2010.09.031
Lv C, Gu T, Ma R, Yao W, Huang Y, Gu J, Zhao G (2021) Biochemical characterization of a GH19 chitinase from Streptomyces alfalfae and its applications in crystalline chitin conversion and biocontrol. Int J Biol Macromol 167:193–201. https://doi.org/10.1016/j.ijbiomac.2020.11.178
Mao X, Guo N, Sun J, Xue C (2017) Comprehensive utilization of shrimp waste based on biotechnological methods: a review. J Clean Prod 143:814–823. https://doi.org/10.1016/j.jclepro.2016.12.042
Mariño MA, Moretti P, Tasic L (2021) Immobilized commercial cellulases onto amino-functionalized magnetic beads for biomass hydrolysis: enhanced stability by non-polar silanization. Biomass Convers Bior, pp 1–11. https://doi.org/10.1007/s13399-021-01798-y
Miller GL (1959) Use of dinitrosaIicyIic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030/
Mohammadzadeh R, Agheshlouie M, Mandavinia GR (2017) Expression of chitinase gene in BL21 pET system and investigating the biocatalystic performance of chitinase-loaded AlgSep nanocomposite beads. Int J Biol Macromol 104:1664–1671. https://doi.org/10.1016/j.ijbiomac.2017.03.119
Moore KG, Price MS, Boston RS, Weissinger AK, Payne GA (2004) A chitinase from Tex6 maize kernels inhibits growth of Aspergillus flavus. Phytopathology 94:82–87. https://doi.org/10.1094/PHYTO.2004.94.1.82
Mukherjee G, Sen SK (2006) Purification, characterization, and antifungal activity of chitinase from Streptomyces venezuelae P10. Curr Microbiol 53:265–269. https://doi.org/10.1007/s00284-005-0412-4
Ngo D, Kim M, Kim S (2008) Chitin oligosaccharides inhibit oxidative stress in live cells. Carbohyd Polym 74:228–234. https://doi.org/10.1016/j.carbpol.2008.02.005
Nishitani Y, Horiuchi A, Aslam M, Kanai T, Atomi H, Miki K (2018) Crystal structures of an archaeal chitinase ChiD and its ligand complexes. Glycobiology 28:418–426. https://doi.org/10.1093/glycob/cwy024
Ohnuma T, Numata T, Osawa T, Inanaga H, Okazaki Y, Shinya S, Kondo K, Fukuda T, Fukamizo T (2012) Crystal structure and chitin oligosaccharide-binding mode of a ‘loopful’ family GH19 chitinase from rye, Secalecereale, seeds. FEBS J 279(19):3639–3651. https://doi.org/10.1111/j.1742-4658.2012.08723.x
Orlando M, Buchholz PCF, Lotti M, Pleiss J (2021) The GH19 Engineering Database: sequence diversity, substrate scope, and evolution in glycoside hydrolase family 19. Plos One 16(10):e256817. https://doi.org/10.1371/journal.pone.0256817
Prasad M, Palanivelu P (2014) A novel method for the immobilization of a thermostable fungal chitinase and the properties of the immobilized enzyme. Biotechnol Appl Bioc 61:441–445. https://doi.org/10.1002/bab.1179
Ricardi NC, Arenas LT, Benvenutti EV, Hinrichs R, Flores EEE, Hertz PF, Costa TMH (2021) High performance biocatalyst based on β-d-galactosidase immobilized on mesoporous silica/titania/chitosan material. Food Chem 359:129890. https://doi.org/10.1016/j.foodchem.2021.129890
Schultz J, Copley RR, Doerks T, Ponting CP, Bork P (2000) SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res 28(1):231–234. https://doi.org/10.1093/nar/28.1.231
Sousa AJS, Silva CFB, Sousa JS, Monteiro Junior JE, Freire JEC, Sousa BL, Lobo MDP, Monteiro-Moreira ACO, Grangeiro TB (2019) A thermostable chitinase from the antagonistic Chromobacteriumviolaceum that inhibits the development of phytopathogenic fungi. Enzyme Microb Tech 126:50–61. https://doi.org/10.1016/j.enzmictec.2019.03.009
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Udaya Prakash NA, Jayanthi M, Sabarinathan R, Kangueane P, Mathew L, Sekar K (2010) Evolution, homology conservation, and identification of unique sequence signatures in GH19 family chitinases. J Mol Evol 70:466–478. https://doi.org/10.1007/s00239-010-9345-z
Wang M, Qi W, Su R, He Z (2015) Advances in carrier-bound and carrier-free immobilized nanobiocatalysts. Chem Eng Sci 135:21–32. https://doi.org/10.1016/j.ces.2015.03.051
Wang W, Guo N, Huang W, Zhang Z, Mao X (2018) Immobilization of chitosanases onto magnetic nanoparticles to enhance enzyme performance. Catalysts 8:401. https://doi.org/10.3390/catal8090401
Watanabe T, Kanai R, Kawase T, Tanabe T, Mitsutomi M, Sakuda S, Miyashita K (1999) Family 19 chitinases of Streptomyces species: characterization and distribution. Microbiology (society for General Microbiology) 145:3353–3363. https://doi.org/10.1099/00221287-145-12-3353
Weber A, Tesch S, Thomas B, Schmiers H (2000) New ways of determining structural groups in brown coals and their bioconversion products by FTIR spectroscopy. Appl Microbiol Biotechnol 54:681–685. https://doi.org/10.1007/s002530000419
Yang S, Fu X, Yan Q, Guo Y, Liu Z, Jiang Z (2016) Cloning, expression, purification and application of a novel chitinase from a thermophilic marine bacterium Paenibacillusbarengoltzii. Food Chem 192:1041–1048. https://doi.org/10.1016/j.foodchem.2015.07.092
Yang S, Fu X, Yan Q, Jiang Z, Wang J (2016) Biochemical characterization of a novel acidic exochitinase from Rhizomucormiehei with antifungal activity. J Agr Food Chem 64:461–469. https://doi.org/10.1016/j.foodchem.2015.07.092
Yano S, Honda A, Rattanakit N, Noda Y, Wakayama M, Plikomol A, Tachiki T (2008) Cloning and expression of chitinase a gene from Streptomyces cyaneus SP-27: the enzyme participates in protoplast formation of Schizophyllum commune. Biosci Biotechnol Biochem 72:1853–1859. https://doi.org/10.1271/bbb.80110
Zhang W, Liu Y, Ma J, Yan Q, Jiang Z, Yang S (2020) Biochemical characterization of a bifunctional chitinase/lysozyme from Streptomyces sampsonii suitable for N-acetyl chitobiose production. Biotechnol Lett 42:1489–1499. https://doi.org/10.1007/s10529-020-02834-z
Zhang W, Ma J, Yan Q, Jiang Z, Yang S (2021) Biochemical characterization of a novel acidic chitinase with antifungal activity from Paenibacillusxylanexedens Z2–4. Int J Biol Macromol 182:1528–1536. https://doi.org/10.1016/j.ijbiomac.2021.05.111
Zheng C, Ding K, Huang X, Yang L, Lei Y, Wang Y (2022) A bioprosthetic heart valve prepared by copolymerization of 2-isocyanatoethyl methacrylate modified pericardium and functional monomer. Compos Part B: Eng 238:109922. https://doi.org/10.1016/j.compositesb.2022.109922
Funding
This work was supported by the earmarked fund for CARS-48.
Author information
Authors and Affiliations
Contributions
F. S., C. X, and X. M. conceived and designed research. A. X. performed research. Y. H. contributed analytical tools. A. X. and Y. H. analyzed data. A. X. and Y. H. wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xing, A., Hu, Y., Wang, W. et al. A novel microbial-derived family 19 endochitinase with exochitinase activity and its immobilization. Appl Microbiol Biotechnol 107, 3565–3578 (2023). https://doi.org/10.1007/s00253-023-12523-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12523-2