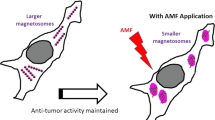
Abstract
We report the fabrication of highly pure magnetosomes that are synthesized by magnetotactic bacteria (MTB) using pharmaceutically compatible growth media, i.e., without compounds of animal origin (yeast extracts), carcinogenic, mutagenic, or toxic for reproduction (CMR) products, and other heavy metals than iron. To enable magnetosome medical applications, these growth media are reduced and amended compared with media commonly used to grow these bacteria. Furthermore, magnetosomes are made non-pyrogenic by being extracted from these micro-organisms and heated above 400 °C to remove and denature bacterial organic material and produce inorganic magnetosome minerals. To be stabilized, these minerals are further coated with citric acid to yield M-CA, leading to fully reconstructed chains of magnetosomes. The heating properties and anti-tumor activity of highly pure M-CA are then studied by bringing M-CA into contact with PC3-Luc tumor cells and by exposing such assembly to an alternating magnetic field (AMF) of 42 mT and 195 kHz during 30 min. While in the absence of AMF, M-CA are observed to be non-cytotoxic, they result in a 35% decrease in cell viability following AMF application. The treatment efficacy can be associated with a specific absorption rate (SAR) value of M-CA, which is relatively high in cellular environment, i.e., SARcell = 253 ± 11 W/gFe, while being lower than the M-CA SAR value measured in water, i.e., SARwater = 1025 ± 194 W/gFe, highlighting that a reduction in the Brownian contribution to the SAR value in cellular environment does not prevent efficient tumor cell destruction with these nanoparticles.
Key points
• Highly pure magnetosomes were produced in pharmaceutically compatible growth media
• Non-pyrogenic and stable magnetosomes were prepared for human injection
• Magnetosomes efficiently destroyed prostate tumor cells in magnetic hyperthermia







Similar content being viewed by others
References
Agranoff DD, Krishna S (1998) Metal ion homeostasis and intracellular parasitism. Mol Microbiol 28:403–412
Alphandéry E (2019a) A discussion on existing nanomedicine regulation: progress and pitfalls. Appl Mater Today 17:193
Alphandéry E (2019b) Iron oxide nanoparticles as multimodal imaging tools. RCS Adv 9:40577
Alphandéry E (2020) Applications of magnetotactic bacteria and magnetosome for cancer treatment: a review emphasizing on practical and mechanistic aspects. Drug Discovery Today 25:1444–1452
Alphandéry E (2021) Light-interacting iron-based nanomaterials for localized cancer detection and treatment. Acta Biomater 124:50–71
Alphandéry E (2022a) Ultrasound and nanomaterial: an efficient pair to fight cancer. Journal of Nanobiotechnology 20:139
Alphandéry E (2022b) Nanomaterials as ultrasound theragnostic tools for heart disease treatment/diagnosis. Int J Mol Sci 23:1683
Alphandéry E, Lijeour L, Lalatonne Y, Motte L (2010) Different signatures between chemically and biologically synthesized nanoparticles in a magnetic sensor: a new technology for multiparametric detection. Sens Actuators, B Chem 147:786
Alphandéry E, Guyot F, Chebbi I (2012) Preparation of chains of magnetosomes, isolated from Magnetospirillum magneticum strain AMB-1 magnetotactic bacteria, yielding efficient treatment of tumors using magnetic hyperthermia. Int J Pharm 434:444–452
Alphandéry E, Abi-Haidar D, Seksek O, Trautmann A, Bergovicci N, Gazeau F, Guyot F, Chebbi I (2017) Nanoprobe synthesized by magnetotactic bacteria, detecting fluorescence variations under dissociation of Rhodamine B from magnetosomes following temperature, pH changes, or the application of radiation. ACS Appl Mater Interfaces 9:36561
Alphandéry E, Idbaih A, Adam C, Delattre J-Y, Schmitt C, Gazeau F, Guyot F, Chebbi I (2019) Biodegraded magnetosomes with reduced size and heating power maintain a persistent activity against intracranial U87-Luc mouse GBM tumors. J Nanobiotechnol 17:126
Alphandéry E, Abi Haidar D, Seksek O, Guyot F, Chebbi I (2018) Fluorescent magnetosomes for controlled and repetitive drug release under the application of an alternating magnetic field under conditions of limited temperature increase (<2.5 °C). Nanoscale 10:10918.
Amstad E, Textor M, Reimhult E (2011) Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 3:2819
Attaluri A, Kandala SK, Wabler M, Zhou H, Cornejo C, Armour M, Hedayati M, Zhang Y, DeWeese TL, Herman C, Ivkov R (2015) Magnetic nanoparticle hyperthermia enhances radiation therapy: a study in mouse models of human prostate cancer. Int J Hyperth 31:359–374
Berny C, Le Fèvre R, Guyot F, Blondeau K, Guizonne C, Rousseau E, Bayan N, Alphandéry E (2020) A method for producing highly pure magnetosomes in large quantity for medical applications using Magnetospirillum gryphiswaldense MSR-1 magnetotactic bacteria amplified in minimal growth media. Front Bioeng Biotechnol 8:16
Carrey J, Mehdaoui B, Respaud M (2011) Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: application to magnetic hyperthermia optimization. J Appl Phys 109:083921
Cheraghipour E, Javadpour S, Mehdizadeh AR (2012) Citrate capped superparamagnetic iron oxide nanoparticles used for hyperthermia therapy. JBiSE 05:715–719
Cortés-Llanos B, Ocampo SM, de la Cueva L, Calvo GF, Belmonte-Beitia J, Pérez L, Salas G, Ayuso-Sacido Á (2021) Influence of coating and size of magnetic nanoparticles on cellular uptake for in vitro MRI. Nanomater 11:28888
Cypriano J, Werckmann J, Vargas G, Lopes dos Santos A, Silva KT, Leão P, Almeida FP, Bazylinski DA, Farina M, Lins U, Abreu F (2019) Uptake and persistence of bacterial magnetite magnetosomes in a mammalian cell line: implications for medical and biotechnological applications. PLoS One 14:e0215657
Dieudonné A, Pignol D, Prévéral S (2019) Magnetosomes: biogenic iron nanoparticles produced by environmental bacteria. Appl Microbiol Biotechnol 103:3637–3649
Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K (2018) Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep 8:2082
Field SB, Bleehen NM (1979) Hyperthermia in the treatment of cancer. Cancer Treat Rev 6:63–94
Geddes LA (1999) d’Arsonval, physician and inventor. IEEE Eng Med Biol Mag 18:118–122
Giustini AJ, Ivkov R, Hoopes PJ (2011) Magnetic nanoparticle biodistribution following intratumoral administration. Nanotechnol 22:345101
Guo L, Huang J, Zheng L-M (2011) Control generating of bacterial magnetic nanoparticle–doxorubicin conjugates by poly-L-glutamic acid surface modification. Nanotechnol 22:175102
Hainfeld J, Huang H (2013) Intravenous magnetic nanoparticle cancer hyperthermia. Int J Nanomedicine 8:2521–2532. https://doi.org/10.2147/IJN.S43770
Hamdous Y, Chebbi I, Mandawala C, Le Fèvre R, Guyot F, Seksek O, Alphandéry E (2017) Biocompatible coated magnetosome minerals with various organization and cellular interaction properties induce cytotoxicity towards RG-2 and GL-261 glioma cells in the presence of an alternating magnetic field. J Nanobiotechnol 15:74
El Hedjaj C, Barret E, Chebbi I, Le Fèvre R, Maake C, Gushetti F, Guyot F, Seksek O, Alphandéry E (in preparation) Full disappearance of subcutaneous mouse PC3-Luc prostate tumors by direct intratumor magnetosome injection followed by 6 to 10 hyperthermia sessions at 45°C under ultrasound applications
Herion JC, Walker RI, Palmer JG (1961) Endotoxin fever in granulocytopenic animals. J Exp Med 113:1115–1125
Heyen U, Schüler D (2003) Growth and magnetosome formation by microaerophilic Magnetospirillum strains in an oxygen-controlled fermentor. Appl Microbiol Biotechnol 61:536–544
Hildebrandt B (2002) The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 43:33–56
Himmelreich U, Dresselaers T (2009) Cell labeling and tracking for experimental models using magnetic resonance imaging. Methods 48:112–124
Hoffman WD, Natanson C (1993) Endotoxin in septic shock. Anesth Analg 77:613–624
ICH (2019) ICH Q3D guideline for elemental impurities. EMA/CHMP/ICH/353369/2013 Committee for Medicinal Products for Human Use
ISO (2009) ISO 10993–5 Biological evaluation of medical devices — Part 5: tests for in vitro cytotoxicity. Technical Committee : ISO/TC 194 Biological and clinical evaluation of medical devices
ISO (2017) ISO 10993–11 Biological evaluation of medical devices — Part 11: tests for systemic toxicity. Technical Committee : ISO/TC 194 Biological and clinical evaluation of medical devices
Johannsen M, Gneveckow U, Eckelt L, Feussner A, WaldÖFner N, Scholz R, Deger S, Wust P, Loening SA, Jordan A (2005) Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int J Hyperth 21:637–647
Jose J, Kumar R, Harilal S, Mathew GE, Parambi DGT, Prabhu A, Uddin MdS, Aleya L, Kim H, Mathew B (2020) Magnetic nanoparticles for hyperthermia in cancer treatment: an emerging tool. Environ Sci Pollut Res 27:19214–19225
Karvelas EG, Karakasidis TE, Sarris IE (2018) Computational analysis of paramagnetic spherical Fe3O4 nanoparticles under permanent magnetic fields. Comput Mater Sci 154:464–471
Katzmann E, Eibauer M, Lin W, Pan Y, Plitzko JM, Schüler D (2013) Analysis of magnetosome chains in magnetotactic bacteria by magnetic measurements and automated image analysis of electron micrographs. Appl Environ Microbiol 79:7755–7762
Kawai N, Ito A, Nakahara Y, Futakuchi M, Shirai T, Honda H, Kobayashi T, Kohri K (2005) Anticancer effect of hyperthermia on prostate cancer mediated by magnetite cationic liposomes and immune-response induction in transplanted syngeneic rats. Prostate 64:373–381
Kirchner D, Henwood S (2012) Toxicity and safety testing. In: The laboratory rabbit, guinea pig, hamster, and other rodents. A volume in American College of Laboratory Animal Medicine (Chapter 12, pp 275–300). Academic press, Elsevier
Krasil’nikov VN, Gyrdasova OI, Tyutyunnik AP, Diachkova TV, Baklanova IV, Marchenkov VV, Domozhirova AN, Bamburov VG (2018) Thermal and magnetic properties of maghemite γ-Fe2O3 synthesized by a precursor method. Dokl Chem 481:161–165
Le Fèvre R, Durand-Dubief M, Chebbi I, Mandawala C, Lagroix F, Valet J-P, Idbaih A, Adam C, Delattre J-Y, Schmitt C, Maake C, Guyot F, Alphandéry E (2017) Enhanced antitumor efficacy of biocompatible magnetosomes for the magnetic hyperthermia treatment of glioblastoma. Theranostics 7:4618–4631
Lefèvre CT, Abreu F, Lins U, Bazylinski DA (2011) A bacterial backbone: magnetosomes in magnetotactic bacteria. In: Rai M, Duran N (eds) Metal nanoparticles in microbiology. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 75–102
LeVeen HH, Ahmed N, Piccone VA, Shugaar S, Falk G (1980) Radio-frequency therapy: clinical experience. Ann NY Acad Sci 335:362–371
Li Y, Boraschi D (2016) Endotoxin contamination: a key element in the interpretation of nanosafety studies. Nanomed 11:269–287
Li J, Pan Y, Chen G, Liu Q, Tian L, Lin W (2009) Magnetite magnetosome and fragmental chain formation of Magnetospirillum magneticum AMB-1: transmission electron microscopy and magnetic observations. Geophys J Int 177:33–42
Mai BT, Balakrishnan PB, Barthel MJ, Piccardi F, Niculaes D, Marinaro F, Fernandes S, Curcio A, Kakwere H, Autret G, Cingolani R, Gazeau F, Pellegrino T (2019) Thermoresponsive iron oxide nanocubes for an effective clinical translation of magnetic hyperthermia and heat-mediated chemotherapy. ACS Appl Mater Interfaces 11:5727–5739
Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A (2011) Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 103:317–324
Mandawala C, Chebbi I, Durand-Dubief M, Le Fèvre R, Hamdous Y, Guyot F, Alphandéry E (2017) Biocompatible and stable magnetosome minerals coated with poly-l-lysine, citric acid, oleic acid, and carboxy-methyl-dextran for application in the magnetic hyperthermia treatment of tumors. J Mater Chem B 5:7644–7660
Mannucci S, Ghin L, Conti G, Tambalo S, Lascialfari A, Orlando T, Benati D, Bernardi P, Betterle N, Bassi R, Marzola P, Sbarbati A (2014) Magnetic nanoparticles from Magnetospirillum gryphiswaldense increase the efficacy of thermotherapy in a model of colon carcinoma. PLoS One 9:e108959
Mannucci S, Tambalo S, Conti G, Ghin L, Milanese A, Carboncino A, Nicolato E, Marinozzi MR, Benati D, Bassi R, Marzola P, Sbarbati A (2018) Magnetosomes extracted from Magnetospirillum gryphiswaldense as theranostic agents in an experimental model of glioblastoma. Contrast Media Mol Imaging 2018:1–12
McCausland HC, Komeili A (2020) Magnetic genes: studying the genetics of biomineralization in magnetotactic bacteria. PLoS Genet 16:e1008499
Milleron RS, Bratton SB (2007) ‘Heated’ debates in apoptosis. Cell Mol Life Sci 64:2329–2333
Mohapatra J, Zeng F, Elkins K, Xing M, Ghimire M, Yoon S, Mishra SR, Liu JP (2018) Size-dependent magnetic and inductive heating properties of Fe3O4 nanoparticles: scaling laws across the superparamagnetic size. Phys Chem Chem Phys 20:12879–12887
Moisescu C, Bonneville S, Staniland S, Ardelean I, Benning LG (2011) Iron uptake kinetics and magnetosome formation by Magnetospirillum gryphiswaldense as a function of pH, temperature and dissolved iron availability. Geomicrobiol J 28:590–600
Molcan M, Kaczmarek K, Kubovcikova M, Gojzewski H, Kovac J, Timko M, Józefczak A (2020) Magnetic hyperthermia study of magnetosome chain systems in tissue-mimicking phantom. J Mol Liq 320:114470
Moskowitz BM, Frankel RB, Bazylinski DA (1993) Rock magnetic criteria for the detection of biogenic magnetite. Earth Planetary Sci Lett 120:283–300
Muthana M, Kennerley AJ, Hughes R, Fagnano E, Richardson J, Paul M, Murdoch C, Wright F, Payne C, Lythgoe MF, Farrow N, Dobson J, Conner J, Wild JM, Lewis C (2015) Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nat Commun 6:8009
Muthukumaran T, Philip J (2014) Enhanced thermal stability of phosphate capped magnetite nanoparticles. J Appl Phys 115:224304
Nemati Z, Alonso J, Rodrigo I, Das R, Garaio E, García JÁ, Orue I, Phan M-H, Srikanth H (2018) Improving the heating efficiency of iron oxide nanoparticles by tuning their shape and size. J Phys Chem C 122:2367–2381
Nguyen KT, Zhao Y (2015) Engineered hybrid nanoparticles for on-demand diagnostics and therapeutics. Acc Chem Res 48:3016–3025
Nigam S, Barick KC, Bahadur D (2011) Development of citrate-stabilized Fe3O4 nanoparticles: conjugation and release of doxorubicin for therapeutic applications. J Magn Magn Mater 323:237–243
Popa R, Fang W, Nealson KH, Souza-Egipsy V, Berquó TS, Benerjee SK, Penn LR (2009) Effect of oxidative stress on the growth of magnetic particles in Magnetospirillum magneticum. Int Microbiol 12:47–58
Premaratne RJ, Lin WJ, Johnson EA (1991) Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl Environ Microbiol 57:3046–3048
Proksch RB, Schäffer TE, Moskowitz BM, Dahlberg ED, Bazylinski DA, Frankel RB (1995) Magnetic force microscopy of the submicron magnetic assembly in a magnetotactic bacterium. Appl Phys Lett 66:2582–2584
Rahn-Lee L, Komeili A (2013) The magnetosome model: insights into the mechanisms of bacterial biomineralization. Front Microbiol 4:352
Rosensweig RE (2002) Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater 252:370–374
Salimi M, Sarkar S, Saber R, Delavari H, Alizadeh AM, Mulder HT (2018) Magnetic hyperthermia of breast cancer cells and MRI relaxometry with dendrimer-coated iron-oxide nanoparticles. Cancer Nano 9:7
Sanz B, Calatayud MP, De Biasi E, Lima E, Mansilla MV, Zysler RD, Ibarra MR, Goya GF (2016) In silico before in vivo: how to predict the heating efficiency of magnetic nanoparticles within the intracellular space. Sci Rep 6:38733
Saville SL, Qi B, Baker J, Stone R, Camley RE, Livesey KL, Ye L, Crawford TM, Thompson Mefford O (2014) The formation of linear aggregates in magnetic hyperthermia: implications on specific absorption rate and magnetic anisotropy. J Colloid Interface Sci 424:141–151
Scheffel A, Gärdes A, Grünberg K, Wanner G, Schüler D (2008) The major magnetosome proteins MamGFDC are not essential for magnetite biomineralization in Magnetospirillum gryphiswaldense but regulate the size of magnetosome crystals. J Bacteriol 190:377–386
Seegenschmiedt MH, Vernon CC (1995) A historical perspective on hyperthermia in oncology. In: Seegenschmiedt MH, Fessenden P, Vernon CC (eds) Thermoradiotherapy thermochemotherapy: biology, physiology, physics. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 3–44
Shah RR, Dombrowsky AR, Paulson AL, Johnson MP, Nikles DE, Brazel CS (2016) Determining iron oxide nanoparticle heating efficiency and elucidating local nanoparticle temperature for application in agarose gel-based tumor model. Mater Sci Eng: C 68:18–29
Stillwell W (2016) Membrane transport. In: An introduction to biological membranes. Academic Press, Elsevier, pp 423–451
Tong S, Quinto CA, Zhang L, Mohindra P, Bao G (2017) Size-dependent heating of magnetic iron oxide nanoparticles. ACS Nano 11:6808–6816
Uebe R, Schüler D (2016) Magnetosome biogenesis in magnetotactic bacteria. Nat Rev Microbiol 14:621–637
Usov NA, Gubanova EM (2020) Application of magnetosomes in magnetic hyperthermia. Nanomater 10:1320
Usov NA, Liubimov BYA (2012) Dynamics of magnetic nanoparticle in a viscous liquid: application to magnetic nanoparticle hyperthermia. J Appl Phys 112:023901
Vargas HI, Dooley WC, Gardner RA, Gonzalez KD, Venegas R, Heywang-Kobrunner SH, Fenn AJ (2004) Focused microwave phased array thermotherapy for ablation of early-stage breast cancer: results of thermal dose escalation. Ann Surg Oncol 11:139–146
Wang X, Quinn PJ (2010) Endotoxins: lipopolysaccharides of gram-negative bacteria. In: Wang X, Quinn PJ (eds) Endotoxins: structure, function and recognition. Springer, Netherlands, Dordrecht, pp 3–25
Yan L, Da H, Zhang S, López VM, Wang W (2017) Bacterial magnetosome and its potential application. Microbiol Res 203:19–28
Ye X, Lin D, Jiao Z, Zhang L (1998) The thermal stability of nanocrystalline maghemite. J Phys d: Appl Phys 31:2739–2744
Zhang Y, Zhang X, Jiang W, Li Y, Li J (2011) Semicontinuous culture of Magnetospirillum gryphiswaldense MSR-1 cells in an autofermentor by nutrient-balanced and isosmotic feeding strategies. Appl Environ Microbiol 77:5851–5856
Zhao Q, Wang L, Cheng R, Mao L, Arnold RD, Howerth EW, Chen ZG, Platt S (2012) Magnetic nanoparticle-based hyperthermia for head & neck cancer in mouse models. Theranostics 2:113–121
Zhou Y-F (2011) High intensity focused ultrasound in clinical tumor ablation. WJCO 2:8
Zhu N, Ji H, Yu P, Niu J, Farooq M, Akram M, Udego I, Li H, Niu X (2018) Surface modification of magnetic iron oxide nanoparticles. Nanomater 8:810
Acknowledgements
We would like to thank the BPI (“banque publique d’investissement, France”), the region of Paris (“Paris Région Entreprise, France”), the French Research Tax Credit program (“crédit d’impôt recherche”), the incubator Paris Biotech Santé, the ANRT (CIFRE 2014/0359, CIFRE 2016/0747, CIFRE 2013/0364, CIFRE 2015/976), the Eurostars programs (Nanoneck-2 E9309 and Nanoglioma E11778), the AIR program (“aide à l’innovation responsible”) from the region of Paris (A1401025Q), the ANR (“Agence Nationale de la Recherche”) Méfisto, as well as the Universities Paris 6 and Paris 11. We also would like to thank the Nomis Foundation and Markus Reinhard for their support.
Author information
Authors and Affiliations
Contributions
TN, IC, and RL contributed to the study conception, design, and data analysis. TN carried out the experiments and collected the data. TN wrote the first version of the manuscript taking into consideration some advice given by IC and FG. EA reviewed and edited the first version of the manuscript. FG participated significantly in the acquisition of transmission electron microscopy data, in the analysis of all the results, and in the writing of the article. TN is a PhD student who has carried out her PhD under the supervision of EA and FG. All the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
TN, IC, RL, and EA declare that they have been working in the start-up Nanobacterie. FG declares that he has no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The affiliation of Nanobacterie includes the two subsidiaries of Nanobacterie: “AlphaOnco Lux, 16, avenue pasteur L-2310 LUXEMBOURG” and “AlphaOnco Swiss, Route de l'Ile-au-Bois 1a, 1870, Monthey, Switzerland”.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, T.N., Chebbi, I., Le Fèvre, R. et al. Non-pyrogenic highly pure magnetosomes for efficient hyperthermia treatment of prostate cancer. Appl Microbiol Biotechnol 107, 1159–1176 (2023). https://doi.org/10.1007/s00253-022-12247-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12247-9