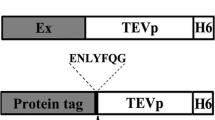
Abstract
The protease catalytic subunit of the nuclear inclusion protein A from tobacco etch virus (TEVp) is widely used to remove tags and fusion proteins from recombinant proteins. Some intrinsic drawbacks to its recombinant production have been studied for many years, such as low solubility, auto-proteolysis, and instability. Some point mutations have been incorporated in the amino acid protease sequence to improve its production. Here, a comprehensive review of each mutation reported so far has been made to incorporate them into a mutant called TEVp7M with a total of seven changes. This mutant with a His7tag at N-terminus was produced with remarkable purification yields (55 mg/L of culture) from the soluble fraction in a single step affinity purification. The stability of His7-TEVp7M was analyzed and compared with the single mutant TEVp S219V, making evident that His7-TEVp7M shows very constant thermal stability against pH variation, whereas TEVp S219V is highly sensitive to this change. The cleavage reaction was optimized by determining the amount of protease that could cleave a 100-fold excess substrate in the shortest possible time at 30 °C. Under these conditions, His7-TEVp7M was able to cleave His-tag in the buffers commonly used for affinity purification. Finally, a structural analysis of the mutations showed that four of them increased the polarity of the residues involved and, consequently, showed increased solubility of TEVp and fewer hydrophobic regions exposed to the solvent. Taken together, the seven changes studied in this work improved stability, solubility, and activity of TEVp producing enough protease to digest large amounts of tags or fusion proteins.
Key points
• Production of excellent yields of a TEVp (TEVp7M) by incorporation of seven changes.
• His-tag removal in an excess substrate in the common buffers used for purification.
• Incorporated mutations improve polarity, stability, and activity of TEVp7M.
Graphical abstract








Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Aslantas Y, Surmeli NB (2019) Effects of N-terminal and C-terminal polyhistidine tag on the stability and function of the thermophilic P450 CYP119. Bioinorg Chem Appl 2019:8080697. https://doi.org/10.1155/2019/8080697
Blommel PG, Fox BG (2007) A combined approach to improving large-scale production of tobacco etch virus protease. Protein Expr Purif 55(1):5368. https://doi.org/10.1016/j.pep.2007.04.013
Brungardt J, Govind R, Trick HN (2020) A simplified method for producing laboratory grade recombinant TEV protease from E coli. Protein Expr Purif. 174:105662. https://doi.org/10.1016/j.pep.2020.105662
Cabrita LD, Gilis D, Robertson AL, Dehouck Y, Rooman M, Bottomley SP (2007) Enhancing the stability and solubility of TEV protease using in silico design. Protein Sci 16(11):2360–2367. https://doi.org/10.1110/ps.072822507
Cesaratto F, López-Requena A, Burrone OR, Petris G (2015) Engineered tobacco etch virus (TEV) protease active in the secretory pathway of mammalian cells. J Biotechnol 212:159–166. https://doi.org/10.1016/j.jbiotec.2015.08.026
Cesaratto F, Burrone OR, Petris G (2016) Tobacco etch virus protease: a shortcut across biotechnologies. J Biotechnol 231:239–249. https://doi.org/10.1016/j.jbiotec.2016.06.012
DeLano WL (2002). Pymol: an open-source molecular graphics tool. CCP4 Newsletter on protein crystallography 40(1): 82–92. http://148.79.162.84/newsletters/newsletter40/11_pymol.pdf
Enríquez-Flores S, Rodríguez-Romero A, Hernández-Alcántara G, Oria-Hernández J, Gutiérrez-Castrellón P, Pérez-Hernández G, de la Mora-de la Mora I, Castillo-Villanueva A, García-Torres I, Méndez ST, Gómez-Manzo S, Torres-Arroyo A, López-Velázquez G, Reyes-Vivas H (2011) Determining the molecular mechanism of inactivation by chemical modification of triosephosphate isomerase from the human parasite Giardia lamblia: a study for antiparasitic drug design. Proteins 79(9):2711–2724. https://doi.org/10.1002/prot.23100
Eisenberg D (1984) Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem 53:595–623. https://doi.org/10.1146/annurev.bi.53.070184.003115
Eisenmesser EZ, Kapust RB, Nawrocki JP, Mazzulla MJ, Pannell LK, Waugh DS, Byrd RA (2000) Expression, purification, refolding, and characterization of recombinant human interleukin-13: utilization of intracellular processing. Protein Expr Purif 20(2):186–195. https://doi.org/10.1006/prep.2000.1283
Fang J, Chen L, Cheng B, Fan J (2013) Engineering soluble tobacco etch virus protease accompanies the loss of stability. Protein Expr Purif 92(1):29–35. https://doi.org/10.1016/j.pep.2013.08.015
Fang L, Jia KZ, Tang YL, Ma DY, Yu M, Hua ZC (2007) An improved strategy for high-level production of TEV protease in Escherichia coli and its purification and characterization. Protein Expr Purif 51(1):102–109. https://doi.org/10.1016/j.pep.2006.07.003
Fraczkiewicz R, Braun W (1998) Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. J Comp Chem 19(3):319–333. https://doi.org/10.1002/(SICI)1096-987X(199802)19:3%3C319::AID-JCC6%3E3.0.CO;2-W
García-Torres I, la Mora-De De, la Mora I, Hernández-Alcántara G, Molina-Ortiz D, Caballero-Salazar S, Olivos-García A, Nava G, López-Velázquez G, Enríquez-Flores S (2018) First characterization of a microsporidial triosephosphate isomerase and the biochemical mechanisms of its inactivation to propose a new druggable target. Sci Rep 8(1):8591. https://doi.org/10.1038/s41598-018-26845-z
Kapust RB, Waugh DS (1999) Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci 8(8):1668–1674. https://doi.org/10.1110/ps.8.8.1668
Kapust RB, Tözsér J, Fox JD, Anderson DE, Cherry S, Copeland TD, Waugh DS (2001) Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng 14(12):993–1000. https://doi.org/10.1093/protein/14.12.993
Kapust RB, Routzahn KM, Waugh DS (2002) Processive degradation of nascent polypeptides, triggered by tandem AGA codons, limits the accumulation of recombinant tobacco etch virus protease in Escherichia coli BL21(DE3). Protein Expr Purif 24(1):61–70. https://doi.org/10.1006/prep.2001.1545
Kondo T, Yumura S (2020) Strategies for enhancing gene expression in Escherichia coli. Appl Microbiol Biotechnol 104(9):3825–3834. https://doi.org/10.1007/s00253-020-10430-4
Kosobokova EN, Skrypnik KA, Kosorukov VS (2016) Overview of fusion tags for recombinant proteins. Biochemistry (mosc) 81(3):187–200. https://doi.org/10.1134/S0006297916030019
Lithwick G, Margalit H (2003) Hierarchy of sequence-dependent features associated with prokaryotic translation. Genome Res 13(12):2665–2673. https://doi.org/10.1101/gr.1485203
Lucast LJ, Batey RT, Doudna JA (2001). Large-scale purification of a stable form of recombinant tobacco etch virus protease. Biotechniques. 30(3). https://doi.org/10.2144/01303st06
Lundbäck AK, van den Berg S, Hebert H, Berglund H, Eshaghi S (2008) Exploring the activity of tobacco etch virus protease in detergent solutions. Anal Biochem 382(1):69–71. https://doi.org/10.1016/j.ab.2008.07.018
Miladi B, Bouallagui H, Dridi C, El Marjou A, Boeuf G, Di Martino P, Dufour F, Elm’Selmi A (2011) A new tagged-TEV protease: construction, optimization of production, purification and test activity. Protein Expr Purif 75(1):75–82. https://doi.org/10.1016/j.pep.2010.08.012
Nallamsetty S, Kapust RB, Tözsér J, Cherry S, Tropea JE, Copeland TD, Waugh DS (2004) Efficient site-specific processing of fusion proteins by tobacco vein mottling virus protease in vivo and in vitro. Protein Expr Purif 38(1):108–115. https://doi.org/10.1016/j.pep.2004.08.016
Nautiyal K, Kuroda Y (2018) A SEP tag enhances the expression, solubility and yield of recombinant TEV protease without altering its activity. N Biotechnol 42:77–84. https://doi.org/10.1016/j.nbt.2018.02.006
Niesen FH, Berglund H, Vedadi M (2007) The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2(9):2212–2221. https://doi.org/10.1038/nprot.2007.321
Nunn CM, Jeeves M, Cliff MJ, Urquhart GT, George RR, Chao LH, Tscuchia Y, Djordjevic S (2005) Crystal structure of tobacco etch virus protease shows the protein C terminus bound within the active site. J Mol Biol 350(1):145–155. https://doi.org/10.1016/j.jmb.2005.04.013
Parks TD, Howard ED, Wolpert TJ, Arp DJ, Dougherty WG (1995) Expression and purification of a recombinant tobacco etch virus NIa proteinase: biochemical analyses of the full-length and a naturally occurring truncated proteinase form. Virology 210(1):194–201. https://doi.org/10.1006/viro.1995.1331
Phan J, Zdanov A, Evdokimov AG, Tropea JE, Peters HK 3rd, Kapust RB, Li M, Wlodawer A, Waugh DS (2002) Structural basis for the substrate specificity of tobacco etch virus protease. J Biol Chem 277(52):50564–50572. https://doi.org/10.1074/jbc.M207224200
Raran-Kurussi S, Tözsér J, Cherry S, Tropea JE, Waugh DS (2013) Differential temperature dependence of tobacco etch virus and rhinovirus 3C proteases. Anal Biochem 436(2):142–144. https://doi.org/10.1016/j.ab.2013.01.031
Renicke C, Spadaccini R, Taxis C (2013) A tobacco etch virus protease with increased substrate tolerance at the P1’ position. PLoS ONE 8(6):e67915. https://doi.org/10.1371/journal.pone.0067915
Rizza JD, Ortega C, Carrión F, Fló M, Correa A (2021) Production, purification and characterization of a double-tagged TEV protease. Protein Expr Purif. 191:106021. https://doi.org/10.1016/j.pep.2021.106021. Epub ahead of print
Shepard JF, Gaard G, Purcifull DE (1974) A study of tobacco etch virus-induced inclusions using indirect immunoferritin procedures. Phytopathology 64:418–425. https://doi.org/10.1094/Phyto-64-418
Sun C, Liang J, Shi R, Gao X, Zhang R, Hong F, Yuan Q, Wang S (2012) Tobacco etch virus protease retains its activity in various buffers and in the presence of diverse additives. Protein Expr Purif 82(1):226–231. https://doi.org/10.1016/j.pep.2012.01.005
Tropea JE, Cherry S, Waugh DS (2009) Expression and purification of soluble His(6)-tagged TEV protease. Methods Mol Biol 498:297–307. https://doi.org/10.1007/978-1-59745-196-3_19
van den Berg S, Löfdahl PA, Härd T, Berglund H (2006) Improved solubility of TEV protease by directed evolution. J Biotechnol 121(3):291–298. https://doi.org/10.1016/j.jbiotec.2005.08.006
Wei L, Cai X, Qi Z, Rong L, Cheng B, Fan J (2012) In vivo and in vitro characterization of TEV protease mutants. Protein Expr Purif 83(2):157–163. https://doi.org/10.1016/j.pep.2012.03.011
Wu X, Wu D, Lu Z, Chen W, Hu X, Ding Y (2009). A novel method for high-level production of TEV protease by superfolder GFP tag. J Biomed Biotechnol. 591923. https://doi.org/10.1155/2009/591923
Zou Z, Cao L, Zhou P, Su Y, Sun Y, Li W (2008) Hyper-acidic protein fusion partners improve solubility and assist correct folding of recombinant proteins expressed in Escherichia coli. J Biotechnol 135(4):333–339. https://doi.org/10.1016/j.jbiotec.2008.05.007
Funding
This work was supported by CONACyT, grant number 259105 and the Recursos Fiscales para Investigación Program from the Instituto Nacional de Pediatría, SS. grant numbers 062/2019, 072/2019, 016/2020.
Author information
Authors and Affiliations
Contributions
Conceptualization: IGT, GLV, SEF; methodology: LAFL, CFL, GHA, CEGB; formal analysis and investigation: SEF, JIDLM, NC, GLV, IGT; writing—original draft preparation: SEF, GLV, IGT. writing—review and editing: CFL, CEGB, IGT; funding acquisition: SEF, GLV, IGT; resources: SEF, JIDLM, LAFL; supervision: GLV, IGT. All authors read and approved the manuscript
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sergio Enríquez-Flores and José Ignacio De la Mora-De la Mora contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Enríquez-Flores, S., De la Mora-De la Mora, J.I., Flores-López, L.A. et al. Improved yield, stability, and cleavage reaction of a novel tobacco etch virus protease mutant. Appl Microbiol Biotechnol 106, 1475–1492 (2022). https://doi.org/10.1007/s00253-022-11786-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11786-5