Abstract
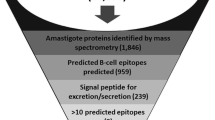
Leishmania braziliensis is responsible for most cases of human tegumentary leishmaniasis (HTL) and has caused a wide range of clinical manifestations, including cutaneous (CL) and mucosal leishmaniasis (ML). The diagnosis is based on criteria that consider epidemiological data, clinical findings, and laboratory tests and is hard to establish. For laboratory tests, none of the assays available can be considered gold standards for disease detection. In addition, the Montenegro skin test, essential to supporting infectologists in the clinical management of the disease, is no longer available in Brazil. Thus, the aim of this study was to develop new targets to be used in diagnostic tests for HTL. In the first step, we carried out two-dimensional gel electrophoresis, followed by mass spectrometry, combined with heat map analysis and immunoproteomics approach, and disclosed eight proteins expressed in the amastigote stage specifically recognized by serum from CL and ML patients. A chimeric protein was designed based on the combination of thirteen linear B-cell epitopes, identified by immunoinformatics analysis, from L. braziliensis proteins. Our results showed that the strategy used in this work was successful in developing an antigen to be used in immunological assays (100.0% sensitivity and specificity) in the detection of HTL cases and in comparison with results obtained from an ELISA using soluble L. braziliensis antigen (SLb-Antigen) and immunofluorescence assay (Bio-Manguinhos/FIOCRUZ). The present technology opens the door for its use in field exams by means of an immunochromatographic test, which will be even more helpful in regions without laboratory structures.
Key points
• Rational strategy to develop antigens.
• Integration between immunoproteomic and immunoinformatics analysis.
• Chimeric protein shows high performance in HTL diagnosis.




Similar content being viewed by others
Data availability
The authors declare that (the/all other) data supporting the findings of this study are available within the article (and its supplementary information files).
References
Aguirre S, Silber AM, Brito ME, Ribone ME, Lagier CM, Marcipar IS (2006) Design, construction, and evaluation of a specific chimeric antigen to diagnose chagasic infection. J Clin Microbiol 44:3768–3774
Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, Team WHOLC (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 7:e35671
Ameen M (2010) Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics. Clin Exp Dermatol 35:699–705
Aslett M, Aurrecoechea C, Berriman M, Brestelli J, Brunk BP, Carrington M, Depledge DP, Fischer S, Gajria B, Gao X, Gardner MJ, Gingle A, Grant G, Harb OS, Heiges M, Hertz-Fowler C, Houston R, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Logan FJ, Miller JA, Mitra S, Myler PJ, Nayak V, Pennington C, Phan I, Pinney DF, Ramasamy G, Rogers MB, Roos DS, Ross C, Sivam D, Smith DF, Srinivasamoorthy G, Stoeckert CJ Jr, Subramanian S, Thibodeau R, Tivey A, Treatman C, Velarde G, Wang H (2010) TriTrypDB: a functional genomic resource for the trypanosomatidae. Nucleic Acids Res 38:D457-462
Boggild AK, Ramos AP, Espinosa D, Valencia BM, Veland N, Miranda-Verastegui C, Arevalo J, Low DE, Llanos-Cuentas A (2010) Clinical and demographic stratification of test performance: a pooled analysis of five laboratory diagnostic methods for American cutaneous leishmaniasis. Am J Trop Med Hyg 83:345–350
Braga Lde S, Navasconi TR, Leatte EP, Skraba CM, Silveira TG, Ribas-Silva RC (2015) Presence of anti-Leishmania (Viannia) braziliensis antibodies in blood donors in the west-central region of the state of Parana, Brazil. Rev Soc Bras Med Trop 48:622–625
Carvalho A, Mendes TAO, Coelho EAF, Duarte MC, Menezes-Souza D (2018) New antigens for the serological diagnosis of human visceral leishmaniasis identified by immunogenomic screening. PLoS One. 13:e0209599
Cataldo JI, de Queiroz Mello FC, Mouta-Confort E, de Fatima MM, de Oliveira SA, da Silva GM, Ribeiro FC, de Fatima M-V, Passos SR (2010) Immunoenzymatic assay for the diagnosis of American tegumentary leishmaniasis using soluble and membrane-enriched fractions from infectious Leishmania (Viannia) braziliensis. J Clin Lab Anal 24:289–294
Coelho VT, Coelho VT, Oliveira JS, Valadares DG, Chávez-Fumagalli MA, Duarte MC, Lage PS, Soto M, Santoro MM, Tavares CA, Fernandes AP, Coelho EA (2012) Identification of proteins in promastigote and amastigote-like Leishmania using an immunoproteomic approach. PLoS Negl Trop Dis. 6:e1430
Cunningham J, Hasker E, Das P, El Safi S, Goto H, Mondal D, Mbuchi M, Mukhtar M, Rabello A, Rijal S, Sundar S, Wasunna M, Adams E, Menten J, Peeling R, Boelaert M, WHO, TDR Visceral Leishmaniasis Laboratory Network. (2012) A global comparative evaluation of commercial immunochromatographic rapid diagnostic tests for visceral leishmaniasis. Clin Infect Dis 55:1312–1319
de Almeida MC, Vilhena V, Barral A, Barral-Netto M (2003) Leishmanial infection: analysis of its first steps. A Review Mem Inst Oswaldo Cruz 98:861–870
de Paiva-Cavalcanti M, de Morais RCS, Pessoa-e-Silva R, Trajano-Silva LAM, Goncalves-de-Albuquerque SD, Tavares DDC, Brelaz-de-Castro MCA, Silva RDE, Pereira VRA. 2015. Leishmaniases diagnosis: an update on the use of immunological and molecular tools. Cell and Bioscience 5
Diniz JL, Costa MO, Gonçalves DU (2011) Mucocutaneous leishmaniasis: clinical markers in presumptive diagnosis. Braz J Otorhinolaryngol 77(3):380–384
Duarte MC, Pimenta DC, Menezes-Souza D, Magalhães RD, Diniz JL, Costa LE, Chávez-Fumagalli MA, Lage PS, Bartholomeu DC, Alves MJ, Fernandes AP, Soto M, Tavares CA, Gonçalves DU, Rocha MO, Coelho EA (2015) Proteins selected in Leishmania (Viannia) braziliensis by an immunoproteomic approach with potential serodiagnosis applications for tegumentary leishmaniasis. Clin Vaccine Immunol 22:1187–1196
Elisei RMT, Matos CS, Carvalho AMRS, Chaves AT, Medeiros FAC, Barbosa R, Marcelino AP, Dos Santos EK, Coelho EAF, Duarte MC, de Oliveira Mendes TA, da Costa Rocha MO, Menezes-Souza D (2018) Immunogenomic screening approach to identify new antigens for the serological diagnosis of chronic Chagas’ disease. Appl Microbiol Biotechnol 102:6069–6080
Gomes-Silva A, Souza MA, Afonso-Cardoso SR, Andrade LR, Dietze R, Lemos E, Belli A, Favoreto Junior S, Ferreira MS (2008) Serological reactivity of different antigenic preparations of Leishmania (Leishmania) amazonensis and the Leishmania braziliensis complex. Rev Soc Bras Med Trop 41:135–141
Gomes CM, de Paula NA, Cesetti MV, Roselino AM, Sampaio RN (2014a) Mucocutaneous leishmaniasis: accuracy and molecular validation of noninvasive procedures in a L. (V.) braziliensis-endemic area. Diagn Microbiol Infect Dis 79:413–418
Gomes CM, Paula NA, Morais OO, Soares KA, Roselino AM, Sampaio RN (2014b) Complementary exams in the diagnosis of american tegumentary leishmaniasis. An Bras Dermatol 89:701–709
Goto H, Lindoso JA (2010) Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther 8:419–433
Grimaldi G Jr, Tesh RB (1993) Leishmaniases of the new world: current concepts and implications for future research. Clin Microbiol Rev 6:230–250
Grimaldi G Jr, Tesh RB, McMahon-Pratt D (1989) A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg 41:687–725
Guimaraes MC, Celeste BJ, Franco EL (1990) Diagnostic performance indices for immunofluorescent tests and enzyme immunoassays of leishmaniasis sera from northern and north-eastern Brazil. Bull World Health Organ 68:39–43
Hailu A (2002) The use of direct agglutination test (DAT) in serological diagnosis of Ethiopian cutaneous leishmaniasis. Diagn Microbiol Infect Dis 42:251–256
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
Indiani de Oliveira C, Teixeira MJ, Teixeira CR, Ramos de Jesus J, Bomura Rosato A, Santa da Silva J, Brodskyn C, Barral-Netto M, Barral A (2004) Leishmania braziliensis isolates differing at the genome level display distinctive features in BALB/c mice. Microbes Infect 6:977–984
Kalter DC (1994) Laboratory tests for the diagnosis and evaluation of leishmaniasis. Dermatol Clin 12:37–50
Kima PE (2014) Leishmania molecules that mediate intracellular pathogenesis. Microbes Infect 16:721–726
Larreta R, Soto M, Quijada L, Folgueira C, Abanades DR, Alonso C, Requena JM (2004) The expression of HSP83 genes in Leishmania infantum is affected by temperature and by stage-differentiation and is regulated at the levels of mRNA stability and translation. BMC Mol Biol 5:3
Machado-Coelho GL, Caiaffa WT, Genaro O, Magalhaes PA, Mayrink W (2005) Risk factors for mucosal manifestation of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg 99:55–61
Malchiodi EL, Chiaramonte MG, Taranto NJ, Zwirner NW, Margni RA (1994) Cross-reactivity studies and differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp; use of immunoblotting and ELISA with a purified antigen (Ag163B6). Clin Exp Immunol 97:417–423
Marcipar IS, Roodveldt C, Corradi G, Cabeza ML, Brito ME, Winter LM, Marcipar AJ, Silber AM (2005) Use of full-length recombinant calflagin and its C fragment for improvement of diagnosis of Trypanosoma cruzi infection. J Clin Microbiol 43:5498–5503
Marsden PD (1986) Mucosal leishmaniasis (“espundia” Escomel, 1911). Trans R Soc Trop Med Hyg 80:859–876
Marzochi MC, Marzochi KB (1994) Tegumentary and visceral leishmaniases in Brazil: emerging anthropozoonosis and possibilities for their control. Cad Saude Publica 10(Suppl 2):359–375
Maurer MH (2016) Two-dimensional gel electrophoresis image analysis via dedicated software packages. Methods Mol Biol 1384:55–65
Mendes TA, Reis Cunha JL, de Almeida Lourdes R, Rodrigues Luiz GF, Lemos LD, dos Santos AR, da Câmara AC, Galvão LM, Bern C, Gilman RH, Fujiwara RT, Gazzinelli RT, Bartholomeu DC (2013) Identification of strain-specific B-cell epitopes in Trypanosoma cruzi using genome-scale epitope prediction and high-throughput immunoscreening with peptide arrays. PLoS Negl Trop Dis. 7:e2524
Menezes-Souza D, Mendes TA, Gomes Mde S, Bartholomeu DC, Fujiwara RT (2015) Improving serodiagnosis of human and canine leishmaniasis with recombinant Leishmania braziliensis cathepsin l-like protein and a synthetic peptide containing its linear B-cell epitope. PLoS Negl Trop Dis. 9:e3426
Menezes-Souza D, de Oliveira Mendes TA, de Araujo Leao AC, de Souza GM, Fujiwara RT, Bartholomeu DC (2015b) Linear B-cell epitope mapping of MAPK3 and MAPK4 from Leishmania braziliensis: implications for the serodiagnosis of human and canine leishmaniasis. Appl Microbiol Biotechnol 99:1323–1336
Menezes-Souza D, Mendes TA, Nagem RA, Santos TT, Silva AL, Santoro MM, de Carvalho SF, Coelho EA, Bartholomeu DC, Fujiwara RT (2014) Mapping B-cell epitopes for the peroxidoxin of Leishmania (Viannia) braziliensis and its potential for the clinical diagnosis of tegumentary and visceral leishmaniasis. PLoS One. 9:e99216
Nandan D, Yi T, Lopez M, Lai C, Reiner NE (2002) Leishmania EF-1alpha activates the Src homology 2 domain containing tyrosine phosphatase SHP-1 leading to macrophage deactivation. J Biol Chem 277:50190–50197
Obuchowski NA, McClish DK (1997) Sample size determination for diagnostic accuracy studies involving binormal ROC curve indices. Stat Med 16:1529–1542
Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S (2007) Cutaneous leishmaniasis. Lancet Infect Dis 7:581–596
Salles BC, Costa LE, Alves PT, Dias AC, Vaz ER, Menezes-Souza D, Ramos FF, Duarte MC, Roatt BM, Chavez-Fumagalli MA, Tavares CA, Goncalves DU, Rocha RL, Goulart LR, Coelho EA (2017) Leishmania infantum mimotopes and a phage-ELISA assay as tools for a sensitive and specific serodiagnosis of human visceral leishmaniasis. Diagn Microbiol Infect Dis 87:219–225
Salotra P, Ralhan R, Bhatnagar R (1994) Differential expression of stress proteins in virulent and attenuated promastigotes of Leishmania donovani. Biochem Mol Biol Int 33:691–697
Saravia NG, Holguin AF, McMahon-Pratt D, D’Alessandro A (1985) Mucocutaneous leishmaniasis in Colombia: Leishmania braziliensis subspecies diversity. Am J Trop Med Hyg 34:714–720
Sarkari B, Ashrafmansouri M, Hatam G, Habibi P, Abdolahi Khabisi S (2014) Performance of an ELISA and indirect immunofluorescence assay in serological diagnosis of zoonotic cutaneous leishmaniasis in iran. Interdiscip Perspect Infect Dis. 2014:505134
Sato CM, Sanchez MC, Celeste BJ, Duthie MS, Guderian J, Reed SG, de Brito ME, Campos MB, de Souza Encarnação HV, Guerra J, de Mesquita TG, Pinheiro SK, Ramasawmy R, Silveira FT, de Assis SM, Goto H (2017) Use of recombinant antigens for sensitive serodiagnosis of American tegumentary leishmaniasis caused by different Leishmania species. J Clin Microbiol 55:495–503
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schubach A, Cuzzi-Maya T, Oliveira AV, Sartori A, de Oliveira-Neto MP, Mattos MS, Araújo ML, Souza WJ, Haddad F, Perez Mde A, Pacheco RS, Momen H, Coutinho SG, de Almeida Marzochi MC, Marzochi KB, da Costa SC (2001) Leishmanial antigens in the diagnosis of active lesions and ancient scars of American tegumentary leishmaniasis patients. Mem Inst Oswaldo Cruz 96:987–996
Silveira FT, Lainson R, Corbett CE (2004) Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem Inst Oswaldo Cruz 99:239–251
SINAN (2018). Sistema de Informação de Agravos de Notificação
Sotto MN, Yamashiro-Kanashiro EH, da Matta VL, de Brito T (1989) Cutaneous leishmaniasis of the new world: diagnostic immunopathology and antigen pathways in skin and mucosa. Acta Trop 46:121–130
Souza AP, Soto M, Costa JM, Boaventura VS, de Oliveira CI, Cristal JR, Barral-Netto M, Barral A (2013) Towards a more precise serological diagnosis of human tegumentary leishmaniasis using Leishmania recombinant proteins. PLoS One. 8:e66110
Srivastava APD, Mehrotra S, Sundar S (2010) Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg 1:6
Sundar S, Rai M (2002) Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol 9:951–958
Team RC (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing
Vexenat Ade C, Santana JM, Teixeira AR (1996) Cross-reactivity of antibodies in human infections by the kinetoplastid protozoa Trypanosoma cruzi, Leishmania chagasi and Leishmania (viannia) braziliensis. Rev Inst Med Trop Sao Paulo 38:177–185
Walker J, Acestor N, Gongora R, Quadroni M, Segura I, Fasel N, Saravia NG (2006) Comparative protein profiling identifies elongation factor-1beta and tryparedoxin peroxidase as factors associated with metastasis in Leishmania guyanensis. Mol Biochem Parasitol 145:254–264
Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A, Moeller S, Schwartz M, Venables B (2020). gplots: various R programming tools for plotting data. R package version 3.0.4. ScienceOpen
Weigle KA, de Davalos M, Heredia P, Molineros R, Saravia NG, D’Alessandro A (1987) Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. Am J Trop Med Hyg 36:489–496
Acknowledgements
MCD, TAOM, EAFC, and DM-S wish to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil, for their fellowships.
Funding
This work was supported by grants from PRPq/UFMG (87–05/2016), CAPES (23038.004862/2015–74), FAPEMIG (APQ-03599–18 and APQ-03821–18), and CNPq (309939/2016–0, 408408/2016–2, 311426/2019–0, and 313070/2018–0).
Author information
Authors and Affiliations
Contributions
DM-S and TAOM conceived and designed research. GCG, AMRS, MCD, MFCS, FACM, and DMMF conducted experiments. DM-S, EAFC, and DTG contributed new reagents or analytical tools. DM-S, TAOM, GCG, and MCD performed result interpretation and data analysis. GCG and DM-S wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The use of human samples was approved by the Ethics Committee of the Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil (protocol CAAE-32343114.9.0000.5149). All patients received an individual copy of the study policy, which was reviewed by an independent person, and all participants signed an informed consent form, in Portuguese, before blood collection. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garcia, G.C., Carvalho, A.M.R.S., Duarte, M.C. et al. Development of a chimeric protein based on a proteomic approach for the serological diagnosis of human tegumentary leishmaniasis. Appl Microbiol Biotechnol 105, 6805–6817 (2021). https://doi.org/10.1007/s00253-021-11518-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11518-1