Abstract
Bioactive compounds from marine environments represent a rich source of bioproducts for potential use in medicine and biotechnology. To discover and identify novel marine natural products (MNPs), evaluating diverse biological activities is critical. Increased sensitivity and specificity of omics technologies, especially next-generation high-throughput sequencing combined with liquid chromatography-mass spectrometry and nuclear magnetic resonance, are speeding up the discovery of novel bioactive compounds. Mycosporine-like amino acids (MAAs) isolated from many marine microorganisms are among highly promising MNPs characterized by ultraviolet radiation (UV) absorbing capacities and are recognized as a potential source of ecologically friendly sunscreens. MAAs absorb damaging UV radiation with maximum absorption in the range of 310–360 nm, including both UVA and UVB ranges. MAAs are also characterized by other biological activities such as anti-oxidant, anti-cancer, and anti-inflammatory activities. The application of modern omics approaches promoted some recent developments in our understanding of MAAs’ functional significance and diversity. This review will summarize the various modern tools that could be applied during the identification and characterization of MNPs, including MAAs, to further their innovative applications.
Key points
• New omics technologies are speeding up the discovery of novel bio-products
• The vast diversity of bioactive capacities of marine natural products described
• Marine microorganisms as a source of environmentally friendly sunscreens
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
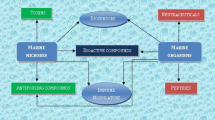
Marine microorganisms are being used in multiple biotechnological applications in industry and for curing diseases. The diversity of marine microorganisms encompasses bacteria (e.g., phyla Actinobacteria and Cyanobacteria), fungi (e.g., phyla Ascomycota and Basidiomycota), microalgae (e.g., phylum Dinoflagellate), diatoms (e.g., phylum Heterokont), and yeast (e.g., the family Saccharomycetaceae). Many novel bioactive molecules have been found in marine microorganisms, including phytoplankton, marine bacteria, cyanobacteria, marine fungi, and dinoflagellates. Recent technological developments, including the application of omics technologies, allowed the exponential increase in the discovery of novel bioproducts. Specifically for marine natural products (MNPs), there is a continual growth in discoveries, with 643 new compounds reported in 2016 (Blunt et al. 2018); 723 in 2017 (Carroll et al. 2019), and even 1884 new bioproducts in 2018 (Carroll et al. 2020). Invertebrates are also at the top of the list of bioproduct valuable organisms, with the second-largest number of new compounds being isolated from the sponge, cnidarians, mollusks, tunicates, and others (Carroll et al. 2019). However, in invertebrates, most MNPs are actually microbe-driven due to their symbiotic relationship with microbial endosymbionts (Jiménez 2018). These MNPs have a wide range of pharmacological activities, including anti-bacterial, anti-viral, anti-malarial, anti-oxidative, antifungal, anti-inflammatory, anti-cancer, anti-allergic, and protective roles such as anti-grazing or anti-fouling. An overview of the major MNP biological activities is provided in Fig. 1, including examples of novel promising bioproducts isolated from marine microorganisms. These roles are important for various industrial applications (Blunt et al. 2018; Carroll et al. 2019, 2020; Rosic 2019), such as in modern trend nutraceuticals and for production of functional foods (Dewapriya and Kim 2014), as well as in the cosmetic industry (Alves et al. 2020).
Diverse activities of marine natural poducts (MNPs) have been reported in numerous compounds isolated from different marine species, including microscopic algae, sea stars, sea urchins, corals, and other microbe-animal symbiotic partnerships (photos done by N. Rosic). The example of novel MNPs includes compounds with antifungal properties such as janthinopolyenemycins isolated from bacteria Janthinobacterium spp. (Anjum et al. 2018). A new compound with anti-bacterial activities kocumarin was isolated from actinobacterium associated with the brown seaweed (Uzair et al. 2018). Neaumycin B isolated from a marine-derived Micromonospora species exhibits potent anti-cancer activity against glioblastoma (Kim et al. 2018b)
Mycosporine-like amino acids (MAAs) are one of the highly promising MNPs found in many marine species. The diversity of these hydrophilic compounds have been reported from micro- and macroalgae to cyanobacteria and a diverse range of marine animals, including invertebrates like corals, sea, urchins, and vertebrates such as fish (Carreto and Carignan 2011; Dunlap and Chalker 1986; Dunlap and Yamamoto 1995; Figueroa 2021; Gröniger et al. 2000; Korbee et al. 2006; Llewellyn and Airs 2010; Orfanoudaki et al. 2020; Sinha et al. 1998). MAAs are characterized by a small mass of less than 400 Da, and a core molecule of either a cyclohexenone or cyclohexenimine ring (Fig. 2) conjugated to an additional molecule such as an amino acid residue or imino alcohol (Garcia-Pichel et al. 1993; Singh et al. 2008b). Over 30 MAAs have been identified so far (Bandaranayake 1998; Llewellyn and Airs 2010; Sinha et al. 1998), ranging from primary to secondary MAAs (Carreto and Carignan 2011; Rastogi et al. 2010). The diversity in MAA composition and UV absorbing capacity have been observed in MAAs isolated from different marine species (Rosic et al. 2015). MAAs are UV-absorbing compounds, with maximum absorption between 310 and 360 nm (Cockell and Knowland 1999). The photo-protective capacities of MAAs are the result of their ability to absorb the light without the production of free radicals in the UVA wavelength range of 315–400 nm (corresponding to ~95% of UV energy that reaches the surface of the Earth) and UVB range of 280–315 nm (Fig. 2).
The impact of UVR on different skin layers (a). Chemical structures of mycosporine-like amino acids (MAAs): (b) MAA core compounds; (c) MAA precursor 4-deoxygadusol, plus commonly found MAAs (mycosporine-glycine, mycosporine-2-glycine, shinorine, palythine, and porphyra-334) including the wavelength of maximum absorbance (λmax)
In addition to the photo-protective role of MAAs, these compounds also act as anti-oxidants and suppressing singlet oxygen-induced damage by scavenging free radicals and other reactive oxygen species (ROS) (De La Coba et al. 2009; Dunlap and Yamamoto 1995; Rastogi et al. 2016). Anti-oxidants (from natural resources and synthetic) are commonly used in modern medicine as bioactive compounds due to their ability to decrease the number of free radicals in cells and tissues (Koltover 2010). Furthermore, MAAs demonstrate multiple biotechnological potentials beyond their UV-protective and anti-oxidative properties (Vanessa Geraldes & Ernani Pinto, 2021). MAAs also exhibit anti-inflammatory capacity (Rosic 2019; Suh et al. 2014), anti-cancer (Fuentes-Tristan et al. 2019; Rajneesh et al. 2017), and other pharmacologically relevant activities (Rojas et al. 2016).
This review will focus on (1) providing an overview of various recently discovered MNPs, with a specific focus on MAA potential for various biotechnological applications, (2) discussing the MNP discovery pipeline that employs novel omics technologies, and finally, (3) debating about some general tools that are employed in the characterization process for new MNPs. Specifically, this review will assess MAA’s biological properties and their potential biotechnological applications.
MNP/MAA biotechnological applications
Natural products are characterized by various biological activities and have been used since ancient times, although they mainly come from terrestrial sources. The chemical structures of nearly half a million natural products are available in different public databases such as PubChem, ZINC, NaprAlert, REAXYS, ChEMBL, and Super Natural II. However, only ~10% of these natural compounds are used commercially (Banerjee et al. 2015; Pereira 2019; Florbela Pereira & Joao Aires-de-Sousa, 2018). Furthermore, MNPs coming from a marine environment, covering ~71% of the Earth’s surface, are not sufficiently used in terms of biotechnological applications. A recently opened MNP database (“Comprehensive Marine Natural Products Database”; https://www.cmnpd.org/) provides detailed information regarding the diversity of MNPs, their physicochemical and pharmacological properties for potential application in drug discoveries (Lyu et al. 2020). So far, the majority of MNPs that have been used in clinical trials and later approved as official drugs were isolated from bacterial and cyanobacterial sources (Pereira and Aires-de-Sousa 2018). The vast potential for the development of new medicines from marine-based sources has been supported by several initiatives in the field of marine biotechnology, including “Blue Growth” of Horizon 2020, which aims to increase the use of marine resources for various biotechnological applications by optimization of cultivation conditions and enhanced sampling processes (Lauritano 2018). Furthermore, the analyses of “Big Data” in chemistry using artificial intelligence and machine learning are a novel promising trend (Tetko and Engkvist 2020). The application of computational methodologies such as chemoinformatics, combining structure-based (SB) and ligand-based (LB) approaches, allowed virtual screening of multiple natural products for computer-assisted drug discovery (Pereira and Aires-de-Sousa 2018).
The MNP pharmacological activities include a diverse range of properties from anti-bacterial, anti-viral, anti-malarial, anti-oxidative, antifungal to anti-inflammatory, anti-cancer, anti-allergic, and anti-grazing or anti-fouling activities, which are important in industrial applications (Blunt et al. 2018; Carroll et al. 2019, 2020; Rosic 2019). However, one of the critical limitations when attempting to discover specific biological activities is the screening process, as other molecules may be required (a type of co-action) for the execution of the biological activity. This mixture of compounds may result in actions being detected only in the mix or when combined with appropriate molecules facilitating the biological activity (Álvarez-Gómez et al. 2019). The examples of different MNPs characterized by various biological properties are provided in Table 1. These compounds show the potential for multifactorial application in biotechnology, specifically in the cosmetic industry for skin protection and anti-aging (Kageyama and Waditee-Sirisattha 2019; Oren and Gunde-Cimerman 2007; Řezanka et al. 2004; Richa and Sinha 2013; Rosic 2019). UV-absorbing MAAs are characterized by multiple biological activities and photo-protective properties that will be further specifically discussed (Alves et al. 2020a, 2020b; Geraldes and Pinto 2021; Huwaidi et al. 2020; Parailloux et al. 2020a, 2020b; Rosic 2019; Singh et al. 2019; Whittock et al. 2020).
Anti-bacterial activities
Anti-microbial resistance (AMR) is an increasing public health problem, impacting people all around the world (WHO 2018a). Due to a rise in the number of antibiotic-resistant strains, there are growing issues around potential complications following major operations or anti-bacterial treatments. For example, multi-drug resistant Mycobacterium tuberculosis causes tuberculosis, which is still one of the deadliest infectious diseases (Parish 2019). Omics technologies were lately applied to search for improved anti-bacterial compounds to address multidrug-resistant bacterial strains and for the development of novel anti-bacterial compounds, which are not toxic to human cells. New compounds isolated from marine microorganisms Zobellia galactanivorans associated with seaweed (e.g., dialkylresorcin and zobelliphol) were discovered by the application of bioinformatics, mass spectrometry (MS), and bioactivity-guided separation processes (Harms et al. 2018). The mechanism of zobelliphol action was confirmed using bacterial reporter strain assays to impact the bacterial DNA biosynthetic processes. Furthermore, a new anti-microbial peptide polyphemusin III (PMIII) was recently isolated from the horseshoe crab Limulus polyphemus (Marggraf et al. 2018). The anti-bacterial activity was confirmed against gram-positive and gram-negative bacteria via cytotoxic activity. By application of recent advances in genomics and proteomics, a recombinant peptide was created and expressed in Escherichia coli, then purified and tested against different bacterial strains, including human cancer cells. Anti-bacterial activity of this β–hairpin peptide was confirmed, as well as additional hemolytic and cytotoxic activities, indicating that bioactive molecules often have multiple pharmacologically relevant properties. Two new antibiotics, branimycins B and C, were isolated through the fermentation of marine bacteria (Pseudonocardia carboxydivorans), living at a 3000 m water depth (Braña et al. 2017). These compounds showed a wide range of anti-bacterial activities inhibiting the growth of 28 different gram-positive and gram-negative bacterial strains.
Using known sequencing information for specific peptides and manipulation on the molecular level resulted in the creation of novel peptides with improved anti-microbial properties (Conceição et al. 2020). The designing of novel bioactive peptides was based on the computational algorithm and produced different analogs that were then tested for physiochemical stability and screened for anti-microbial properties. This recently developed methodological approach in the creation of new anti-microbial peptides is a promising approach for the creation of further anti-bacterial applications. Furthermore, the directed evolution (molecular evolution in vitro) approach, including the so-called DNA shuffling method, was proposed to be a highly efficient tool in creating additional chemical biodiversity and increasing the bioactivity potential of existing natural products (Arnold 2018; Rosic 2009). By using this directed evolution approach, it was possible to engineer novel antibiotics (Cebria-Mendoza et al. 2019), predict AMR (Orencia et al. 2001), or create completely novel protein properties (Rosic 2013).
MAA anti-bacterial activity has not been reported so far, which is in some way the expected outcome as many marine microorganisms produce these secondary metabolites. Therefore, the application of antibiotics may actually have a negative effect on MAA production as anti-bacterial compounds would inhibit the microbial growth and biosynthesis of MAAs. For example, bacteriostatic antibiotics such as chloramphenicol in freshwater copepods cultured conditions resulted in reduced MAA bioaccumulation, confirming the microbial origin of their MAAs (García et al. 2010).
Anti-cancer activities
Based on the World Health Organization, cancer is one of the leading causes of death globally and in 2018, approximately ~9.6 million people died from cancer (WHO 2018b). The increasing number of cancer cases worldwide is partially due to population growth and an increase in the aging population (WCRF 2018). Consequently, there is an apparent increase in the demand for more efficient cancer treatments, and the use of MNPs presents one of the options to be explored further for novel anti-cancer drugs. For evaluating the compounds with anti-cancer or anti-tumor properties, human cancer cell lines such as human breast and cervix (HeLa) cancers and HL60 are often used during screening and efficiency analyses. The examples of potential novel anti-cancer compounds include lipopeptides (i.e., jahanene and jahanane), which were recently isolated from marine cyanobacteria (Okeania sp.) and were found to inhibit cancer cell growth (Iwasaki et al. 2018). The structures of these compounds were established by the application of proteomic methodologies, including nuclear magnetic resonance (NMR) and MS. However, the variability in the level of their anti-cancer effectiveness was found to be impacted by the molecular conformation, which was influenced by the level of unsaturation at the end of the fatty acid molecule (Iwasaki et al. 2018). This presents a challenge that will be important to be addressed for improved consistency if these compounds are selected to be used in anti-cancer therapy.
Recent testing on rats found that compound bryostatin-1, isolated from marine invertebrate Bryozoan, could potentially treat colorectal cancer by reducing oxidative stress and delaying cellular proliferation (Salim et al. 2018). Similarly, numerous other bioactive compounds with anti-cancer properties, which were initially isolated from marine animals, were confirmed to be produced by their bacterial endosymbionts by application of genomic tools for analyses of the biosynthetic pathways (Kwan et al. 2012; Schofield et al. 2015). Multiple compounds recently isolated and characterized from marine organisms demonstrated the potential for the treatment of breast cancer, the most common cancer type in women (WCRF 2018). The newly isolated guaiane sesquiterpene derivative from green algae showed a dose-dependent cytotoxic effect on the triple-negative breast cancer (TNBC) cell line (Martin et al. 2014). Pseudopterosin isolated from the sea whip (the genus Antillogorgia) was found to inhibit the growth of TNBC cells by switching off glucocorticoid receptor α (Sperlich and Teusch 2018), while a cyanobacterial metabolite grassystatin F also demonstrated anti-cancer activity on TNBC cells (Al-Awadhi et al. 2017).
MAA anti-cancer activity was confirmed via inhibition of cancer cell proliferation (Chrapusta et al. 2017). For example, different MAAs isolated from the red algae species Palmaria palmata resulted in dose-dependent inhibition of melanoma cells’ growth (Yuan et al. 2009). Two methanol extracts were isolated from algae exposed to UV-low and UV-high environments, containing multiple MAAs (Fig. 2): palythine, shinorine, asterina-330, palythinol, and porphyra-334; and usujirene (only at high UV). Both extracts showed similar oxygen radical absorbance capacity (ORAC). In contrast, the anti-proliferative capacity of the sample exposed to higher UV was greater, indicating how variation in external conditions could modify the bioactive properties coming from the same organism. A similar anti-proliferative effect was reported with other cancer cell lines (Yuan et al. 2009). MAA isolated from different red algae species also showed a dose-dependent negative impact on HeLa cancer cells via activation of apoptotic mechanisms (Athukorala et al. 2016).
Anti-viral activities
Discovery attempts for new anti-viral compounds have mainly targeted the activities against anti-human immunodeficiency virus (HIV) and human herpes viruses (HHVs) (Asai and Nakashima 2018). The current outbreak of coronavirus disease (COVID-19), due to the action of the severe acute respiratory syndrome (SARS)-like coronavirus-2 (SARS-CoV-2), highlighted the potential of using MNPs for their anti-viral properties, as the development of protective treatments could help in reducing the devastating impact of the current pandemic. New MNPs such as lambda-carrageenan, sulfated polysaccharides, isolated from marine red algae, were found to efficiently inhibit influenza A and B viruses and SARS-CoV-2 (Jang et al. 2021). Spirostaphylotrichin X isolated from marine fungus Cochliobolus lunatus also showed anti-viral activity by inhibiting influenza A virus replication (Wang et al. 2018). Neoechinulin B (NeoB) is another marine fungus-driven compound that exhibits anti-viral properties against hepatitis C virus (HCV) cells in vitro (Nakajima et al. 2016). The NeoB activity against HCV was tested on human hepatocytes and resulted in the reduction of infection. Furthermore, NeoB showed a negative effect on influenza viruses, as it bound to the viral hemagglutinin envelope and disrupted the influenza viruses’ attachment to the host cells (Chen et al. 2015a). Stachybotrin D is a phenylspirodrimane compound isolated from the marine sponge-associated fungus Stachybotrys chartarum that had an inhibitory effect on HIV replication (Ma et al. 2013). Furthermore, there are many promising MNPs with anti-viral properties like Nortopsentin alkaloids, which also demonstrate antifungal and insecticidal activities (Ji et al. 2018). As a result, additional nortopsentin derivatives were designed, produced, and characterized for anti-viral, for phytotoxic, and also for physicochemical properties using biological assays and proton-nuclear magnetic resonance spectroscopy (1H-NMR). These alkaloids have been proposed for further drug development as competent anti-viral agents.
Anti-viral activity has not yet been confirmed or tested for MAA compounds.
Anti-allergic activities
During the last decades, a rise in the number of allergic diseases was reported around the world (Sicherer 2011; Research and Markets 2020). The frequent prevalence of allergy, including specific diseases such as allergic rhinitis, allergic conjunctivitis, asthma, atopic eczema, food allergy, and other allergic conditions in the population, is becoming a huge problem for the health system. Anaphylaxis, an allergic reaction, can be triggered by food, insect bites, or medications, with potentially fatal consequences (Turner et al. 2020). Based on recent business analyses, the allergy treatment market is rapidly increasing and new drugs are urgently required to be developed (Research and Markets 2020). The marine environment may provide some resourceful options. The bicyclic peptides, seongsanamides A–D, extracted from marine bacteria, exhibit significant anti-allergic activity through the inhibition of degranulation and generation of mast cells (Kim et al. 2018a). Butyrolactone I, isolated from the deep-sea-derived Aspergillus species, exhibited anti-allergic capacity by decreasing the number of mast cells in the spleen and some lymph nodes (Liu et al. 2018). From another deep-sea-derived species, actinomycete Nesterenkonia flava were isolated cyclo(d)-Pro-(d)-Leu and indol-3-carbaldehyde compounds that showed promising anti-allergic activity based on immunoglobulin E-mediated effects on rat mast cells (Xie et al. 2017). Based on the total polyphenol content and assessment using the hyaluronidase inhibition assay, in the five marine algae analyzed, the highest anti-allergic activity was detected in Scytosiphon sp. (Chen et al. 2015b). Marine algae were found to be a promising source of MNPs with anti-inflammatory and anti-allergic properties (Vo et al. 2012).
MAA anti-inflammatory activity was also observed for some MAA compounds (Rosic 2019). Mycosporine-glycine and mycosporine-2-glycine, porphyra-334, and shinorine showed an anti-inflammatory effect under certain conditions. When immortal human keratinocytes, the HaCAT cell, were exposed to UV radiation, anti-inflammatory activity was detected for mycosporine-glycine and shinorine, but not porphyra-334 (Suh et al. 2014). On the other hand, both analyzed MAAs, porphyra-334 and shinorine, demonstrated anti-inflammatory activity on human myelomonocytic cells (Becker et al. 2016). These results indicate the variability in MAA bioactive properties under various conditions and cellular environments and the potential for applications in anti-allergic treatments.
MNPs with photo-protective potential for novel sunscreens
Ultraviolet radiation (UVR) levels reaching the Earth’s surface are predicted to increase during this century further (Watanabe et al., 2011). Excessive UVR exposure leads to the development of >95% of human skin cancers (Trager et al. 2020) due to UV-induced DNA damage either directly or indirectly via the production of free radicals (Bertram and Hass 2008). A high-energy UVB radiation has a highly mutagenic and carcinogenic effect resulting in direct DNA damage (Ichihashi et al. 2003). However, through oxidative stress, both UVA and UVB radiation may damage DNA, whereas the ozone layer and atmosphere completely remove the most mutagenic and dangerous UVC. The longer wavelengths, UVA, penetrate deeper, reaching the dermis skin layer, while the shorter wavelengths of UVB, known as burning rays, reach only the epidermal skin layer of skin and are absorbed mostly by keratinocytes (Fig. 2). In humans, the pigment melanin protects the skin from UVR via its UV-absorbing and anti-oxidant properties (Brenner and Hearing 2008). Two forms of melanin pigments are found in the human body and are characterized by some different features. Pheomelanin (orange/yellow pigment) is photosensitizing, resulting in ROS production and potentially DNA damage. On the other hand, eumelanin (brown/black insoluble pigment) is photo-protective (Napolitano et al. 2014). Superoxide and nitric oxide produced by pheomelanin can damage DNA even 2–3 hours after UV exposure due to increased CPD formation (Premi et al. 2015). Importantly, via melanin, human skin will only absorb 50–70% of UVR (Brenner and Hearing 2008). Consequently, human skin needs additional UV protection via externally applied sunscreens. However, current chemical UVR protection is not adequate because commercially available sunscreens lack photostability and can produce free radicals leading to further skin damage, irritation, and allergic reactions (Gaspar et al. 2008; Gaspar and Maia Campos 2006; Greenspoon et al. 2013; Kawakami and Gaspar 2015). In addition, the UV-filter compounds (i.e., oxybenzone and benzophenone-3) used in cosmetic products (within sunscreens) and in the packaging industry, usually reach wastewater and groundwater, resulting in the destruction of ecosystems and environmental pollution (Chaves Lopes et al. 2020; Sánchez-Quiles et al. 2020; Sharifan et al. 2016). As the level of UVR reaching the surface of the Earth will continue to rise, it is critically important to get enhanced UV protection, and using natural marine sunscreens like MAAs presents an exciting opportunity to be explored further.
MAAs isolated from many marine microorganisms are among the most promising MNPs characterized by ultraviolet radiation (UV) absorbing capacities and are recognized as a potential source of ecologically friendly sunscreens (Fig. 2). MAAs are found in numerous marine and freshwater species, including cyanobacteria, algae (i.e., macro-algae and micro-algae), fungi, and higher-order animals such as cnidaria, fishes, echinoderms, mollusks, arthropods, rotifers, and tunicates (Rosic and Dove 2011; Sinha et al. 2007). MAAs are excellent candidates for use in the cosmetic industry and for improved skin protection in an ecologically friendly way. MAAs are water-soluble and highly diverse compounds characterized by photostability and good heat tolerance. The ability of MAAs’ to change their physicochemical and biological properties through modification of their side-chain, including their UV spectral and anti-oxidant capacities, is an exciting opportunity for various biotechnological applications. Additional anti-inflammatory, anti-proliferative, and anti-aging properties could be further utilized for more efficient skin UV protection and skin cancer prevention. MAA biosynthesis is modifiable due to changes happening in the environment (i.e., UVR levels). A better understanding of MAA biosynthesis will permit bigger industry and cosmetics applications in the future. The application of modern omics approaches could be further used to promote our understanding of MAAs’ functional significance and diversity.
The discovery pipeline employing novel omics technologies
The application of omics technologies contributed to the significant increase in the number of new discoveries and added a new layer of complexity and power when analyzing novel compounds (Ambrosino et al. 2019). Genomic, transcriptomic, proteomic, metatranscriptomic, and metabolomic tools have substantially contributed to the processes of MNP discoveries and characterizations during the last decade (Ambrosino et al. 2019; Lauritano et al. 2019), with some representative examples provided in Table 2. The extraction methodology used can influence the molecular structures of isolated compounds and, consequently, their biological activity. Furthermore, the biological extracts often contain multiple compounds, and to maximize their simultaneous separation and detection, it is crucial to complete analyses in a fast, accurate, and comprehensive way minimizing the potential degradation of unstable molecules and contaminations.
After decades of using Sanger sequencing methodology, the fields of genomics and transcriptomics exploring genes and corresponding RNA (or transcripts) start using next-generation sequencing (NGS). NGS allows fast and efficient ways to analyze millions of DNA fragments simultaneously. The application of bioinformatics pipelines via transcriptomics and proteomics has been proven to allow for quicker identification and functional characterization of new MNP such as marine toxins (Xie et al. 2017). For exploring potentially useful venoms to be used as pharmaceuticals, the powerful combination of NGS and liquid chromatography-tandem mass spectrometry resulted in improved sensitivity and discovery of novel venom proteins as potential candidates for drug discovery (Fry et al. 2010). Proteomics analyses, using two-dimensional gel electrophoresis, combined with MALDI-TOF/TOF resulted in the identification of 413 proteins extracted from the sea anemone Bunodactis verrucosa (Domínguez-Pérez et al. 2018). On the contrary, in sea anemone B. verrucose, using the old methodology such as gel-based analyses only eight proteins were identified. Similarly, using a multidimensional approach and combining liquid chromatography with MALDI-TOF-MS resulted in improved separation of compounds extracted from sea anemone Phymanthus crucifer (Rodríguez et al. 2012). This approach allowed for peptide fingerprinting of the sea anemone exudate with 504 different acidic and basic peptides, extending the discovery of novel bioactive compounds.
In metabolomics, the comprehensive exploration of metabolites within biological sample analyses is done using multiple platforms, combining liquid chromatography-mass spectrometry (LC-MS)-based metabolomics (Clish 2015; Kuehnbaum and Britz-McKibbin 2013). The measurement of molecules in this evolving technology is done based on their physical properties and separated metabolites based on polarity, functional groups, or chemical structure (Kuehnbaum and Britz-McKibbin 2013). A variety of metabolomic profiles were detected in three genotypes of the coral Acropora cervicornis, using proton-nuclear magnetic resonance spectroscopy (1H-NMR) and LC-MS metabolomics profiling (Lohr et al. 2019). Specific metabolite profiles were identified, which indicated the differences in protein pathways due to intraspecific variability within coral species. Furthermore, analyzing 13 diatom species via a lipidomics platform that combined an Ultra-Performance Liquid Chromatography (UPLC) and a high resolution/high mass accuracy mass spectrometer made it possible to identify and annotate 142 different lipid compounds (Bromke et al. 2015).
In metatranscriptomics, multiple gene transcripts were assessed, which were produced by microbe-host-based interactions. This approach is helpful as most MNPs are found to be a result of interaction microbe-driven products from symbiotic interactions with other marine organisms (Jiménez 2018), while changes in gene expression are useful for detecting modified pathways important for in bioactive product production. The metatranscriptome approach was also efficiently applied to estimate the variation in ocean microbial communities (Salazar et al. 2019). Consequently, this approach will be useful when combined with already established proteomics and metabolomics pipelines to improve the estimate of novel bioactive product profiles.
Environmental factors impact the biosynthesis of important bioactive molecules
Changes in the external environment are inevitable affecting omics profiles of different marine species. The sequences of hologenomes of ten coral species were recently used to better understand physiological and adaptational changes (Voolstra et al. 2015), with the potential to be used to extend biotechnological capacities of the diverse marine environment. Applying various external conditions may allow for improved and more extensive use of natural biodiversity for the production of desired metabolites (Bode et al. 2002; Dewapriya and Kim 2014). In addition to improving the discovery pathways of novel compounds and increasing the yield of isolated compounds, the culturing conditions could be modified to include the variability in the external conditions (Bode et al. 2002; Figueroa et al. 2014). For example, this may be possible in groups such as marine microalgae, which are a highly potent source of biologically active molecules, are easy to cultivate, have a short generation time, and represent an environmentally friendly option (Lauritano et al. 2018). The screening of 32 microalgal species for anti-bacterial, anti-oxidant, anti-inflammatory, anti-cancer, and anti-diabetes activities, under various culturing conditions, including nitrogen and phosphate-limited environments, resulted in considerable changes in biological activities (Lauritano et al. 2016). Similarly, microalgae under changed light conditions, temperature, modified salinity, and nutrient conditions produce various compounds (Ingebrigtsen et al. 2016; Lauritano et al. 2016) and can adapt to changes happening in the external environment (Dewapriya and Kim 2014. The improved anti-tuberculosis activity was observed in two diatoms only when culturing was done under control and phosphate–starvation conditions, but not under low nitrogen conditions (Lauritano et al. 2018). Consequently, nature’s bioproduct diversity can be further utilized through the manipulation of environmental conditions combined with emerging omics technologies for improved detection and screening.
MAAs are synthesized through the major four-enzyme MAA pathway identified in cyanobacteria (Balskus and Walsh 2010). However, a genetic discrepancy has been reported with the three-gene shinorine pathway also found in many organisms (D'Agostino et al. 2019; Miyamoto et al. 2014). The main variability was reported regarding the presence/absence of certain discrete enzymes, which were encoded by mysE and mysD genes (D'Agostino et al. 2019; Gao and Garcia-Pichel 2011). The presence of genetic diversity within genes from MAA pathways among marine species (Rosic 2019) indicates the additional potential for the discrepancy in regulatory processes during MAA biosynthesis among various species that still need to be explored further. Consequently, these are significant gaps in understanding the genetic variability and mechanisms of gene regulations of the MAA biosynthetic pathway. However, the application of multiple omics approaches may help in better understanding of regulatory mechanisms of MAA synthesis important for improving the discovery options of biotechnologically important MAAs. Some examples of omics applications used include a transcriptome mining approach to determine new MAA pathway genes (Rosic 2012), while proteomic dataset analyses revealed UV-stimulated MAA synthesis mainly via the shikimate pathway (Pope et al. 2015). Using transcriptomic data, the impact UV and far-red lights on transcriptional regulation of MAA were evaluated (Llewellyn et al. 2020), and the MAA gene counterparts were characterized for different microalgal species (Rosic 2012). Recently, a metabolomics approach via Hydrophilic Interaction Liquid Chromatography (HILIC)-Electrospray Orbitrap MS2/MS3 was used to explore MAAs in four algae and resulted in the discovery of 23 new, previously non-reported MAAs (Parailloux, 2020). This study should encourage more extensive use of omics technologies for the exploration of MAAs.
In MAAs, depending on the external conditions and extraction methods used, specific pharmacological activities could be further enhanced or diminished when manipulated under strictly controlled biotechnological processes. The general MAA extraction protocol can utilize ice-cold methanol for MAA extraction from different species (e.g., red algae and microalgae isolated from the cnidarian host) applied HPLC and LC-MS (Fig. 3) to confirm the identity of isolated compounds (Rosic et al. 2015). However, variations in MAA profiles due to changes in environmental conditions have been reported for various marine species (Oren and Gunde-Cimerman 2007; Singh et al. 2008a; Waditee-Sirisattha et al. 2015; Waditee-Sirisattha et al. 2014). The biotechnological manipulations, including variations in the abiotic factors such are temperature, salinity, and light conditions, resulted in variations in the MAA levels and profiles (Ingebrigtsen et al. 2016) and were affected by seasonal fluctuations (Al-Utaibi et al. 2009). The light levels and spectra, specifically UV conditions, temperature conditions, water acidity, and salinity, had a critical influence on quantities and qualities of MAAs (Rosic 2019; Tartarotti and Sommaruga 2006). UV radiation has been found to act as a major factor influencing the MAA accumulation and resulting in changes to the organisms’ MAA profile (Yakovleva and Baird 2005). Spectral variability and intensity were found to affect the synthesis of MAAs (Rastogi et al. 2010). Although MAA production was mainly induced by exposure to UVR (Portwich and Garcia-Pichel 1999), blue light within photosynthetically active radiation together with UVB also had a significant impact on MAA synthesis (Hernando et al. 2002; Sinha et al. 2003). The positive impact on MAA biosynthesis was reported to some extent to exposure to far-red light suggesting the potential role of MAA in the process of thermoregulation (Llewellyn et al. 2020). Consequently, external factors can be further adjusted to maximize MAA production in vivo and maximize MAA therapeutic potentials and biotechnological applications.
The HPLC-MS chromatogram at 330 nm of a methanol extract of the red alga Acanthophora spicifera (adapted from Rosic et al. 2015). Total ion chromatogram (TIC) that includes a summary of intensity for the mass range of m/z 200–450 of positive ions targeting the masses of known MAAs was applied for MAA peak identification based on retention time, absorption maxima, and m/z of positively charged ions [M+H]+
Conclusion and further perspectives
The application of new technologies such as omics technologies provides more efficient ways to obtain novel bioactive compounds. Combining data from various sources and databases, including transcriptomes, proteomes, metabolomes, and epigenomes, will speed up the discovery of new MNPs, their biosynthetic pathways, and prospective biotechnological applications. MAAs, as one of the highly promising MNPs, are excellent candidates for use in the cosmetic industry and for improved skin protection in an ecologically friendly way. The ability of MAAs to change their physicochemical and biological properties due to UVR and exposure to other environmental stressors resulting in improved photo-protective, anti-oxidant, and anti-inflammatory capacities is highly propitious for various biotechnological applications. Improving our understanding of the regulatory mechanisms dictating MAA biosynthesis via the application of novel omics technologies will get us a step closer to the use of MAAs on a bigger industry scale and will unlock numerous biotechnological potentials for these MNPs in the future.
Change history
22 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00253-021-11604-4
References
Al-Awadhi FH, Law BK, Paul VJ, Luesch H (2017) Grassystatins D–F, potent aspartic protease inhibitors from marine Cyanobacteria as potential antimetastatic agents targeting invasive breast cancer. J Nat Prod 80(11):2969–2986. https://doi.org/10.1021/acs.jnatprod.7b00551
Al-Utaibi AA, Niaz GR, Al-Lihaibi SS (2009) Mycosporine-like amino acids in six scleractinian coral species. Oceanologia 51(1):93–104. https://doi.org/10.5697/oc.51-1.093
Álvarez-Gómez F, Korbee N, Casas-Arrojo V, Abdala-Díaz RT, Figueroa FL (2019) UV photoprotection, cytotoxicity and immunology capacity of red algae extracts. Molecules 24(2). https://doi.org/10.3390/molecules24020341
Alves A, Sousa E, Kijjoa A, Pinto M (2020) Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules 25(11). https://doi.org/10.3390/molecules25112536
Ambrosino L, Tangherlini M, Colantuono C, Esposito A, Sangiovanni M, Miralto M, Sansone C, Chiusano ML (2019) Bioinformatics for marine products: an overview of resources, bottlenecks, and perspectives. Mar Drugs 17(10). https://doi.org/10.3390/md17100576
Anjum K, Sadiq I, Chen L, Kaleem S, Li X-C, Zhang Z, Yuan X (2018) Novel antifungal janthinopolyenemycins A and B from a co-culture of marine-associated Janthinobacterium spp. ZZ145 and ZZ148. Tetrahedron Lett 59. https://doi.org/10.1016/j.tetlet.2018.08.022
Arnold FH (2018) Directed evolution: bringing new chemistry to life. Angew Chem Int Ed Eng 57(16):4143–4148. https://doi.org/10.1002/anie.201708408
Asai D, Nakashima H (2018) Pathogenic viruses commonly present in the oral cavity and relevant antiviral compounds derived from natural products. Medicines (Basel, Switzerland) 5(4):120. https://doi.org/10.3390/medicines5040120
Athukorala Y, Trang S, Kwok C, Yuan YV (2016) Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible Canadian marine red macroalgae. Molecules 21(1):E119. https://doi.org/10.3390/molecules21010119
Balskus EP, Walsh CT (2010) The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 329(5999):1653–1656. https://doi.org/10.1126/science.1193637
Bandaranayake WM (1998) Mycosporines: are they nature’s sunscreens? Nat Prod Rep 15(2):159–172
Banerjee P, Erehman J, Gohlke BO, Wilhelm T, Preissner R, Dunkel M (2015) Super natural II--a database of natural products. Nucleic Acids Res 43(Database issue):D935–D939. https://doi.org/10.1093/nar/gku886
Becker K, Hartmann A, Ganzera M, Fuchs D, Gostner JM (2016) Immunomodulatory effects of the mycosporine-like amino acids shinorine and porphyra-334. Mar Drugs 14(6):119 https://www.mdpi.com/1660-3397/14/6/119
Bertram C, Hass R (2008) Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol Chem 389(3):211–220. https://doi.org/10.1515/BC.2008.031
Blunt JW, Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR (2018) Marine natural products. Nat Prod Rep 35(1):8–53. https://doi.org/10.1039/c7np00052a
Bode HB, Bethe B, Höfs R, Zeeck A (2002) Big effects from small changes: possible ways to explore nature's chemical diversity. Chembiochem 3(7):619–627. https://doi.org/10.1002/1439-7633(20020703)3:7<619::Aid-cbic619>3.0.Co;2-9
Braña AF, Sarmiento-Vizcaíno A, Pérez-Victoria I, Otero L, Fernández J, Palacios JJ, Martín J, de la Cruz M, Díaz C, Vicente F, Reyes F, García LA, Blanco G (2017) Branimycins B and C, Antibiotics produced by the abyssal actinobacterium Pseudonocardia carboxydivorans M-227. J Nat Prod 80(2):569–573. https://doi.org/10.1021/acs.jnatprod.6b01107
Brasseur L, Hennebert E, Fievez L, Caulier G, Bureau F, Tafforeau L, Flammang P, Gerbaux P, Eeckhaut I (2017) The roles of spinochromes in four shallow water tropical sea urchins and their potential as bioactive pharmacological agents. Mar Drugs 15(6). https://doi.org/10.3390/md15060179
Brenner M, Hearing VJ (2008) The protective role of melanin against UV damage in human skin. Photochem Photobiol 84(3):539–549. https://doi.org/10.1111/j.1751-1097.2007.00226.x
Bromke MA, Sabir JS, Alfassi FA, Hajarah NH, Kabli SA, Al-Malki AL, Ashworth MP, Méret M, Jansen RK, Willmitzer L (2015) Metabolomic profiling of 13 diatom cultures and their adaptation to nitrate-limited growth conditions. PLoS ONE 10(10):e0138965–e0138965. https://doi.org/10.1371/journal.pone.0138965
Carreto JI, Carignan MO (2011) Mycosporine-like amino acids: relevant secondary metabolites. Chemical and ecological aspects. Mar Drugs 9(3):387–446. https://doi.org/10.3390/md9030387
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR (2019) Marine natural products. Nat Prod Rep 36(1):122–173. https://doi.org/10.1039/c8np00092a
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR (2020) Marine natural products. Nat Prod Rep 37(2):175–223. https://doi.org/10.1039/c9np00069k
Cebria-Mendoza M, Sanjuan R, Domingo-Calap P (2019) Directed Evolution of a Mycobacteriophage. Antibiotics (Basel) 8(2). https://doi.org/10.3390/antibiotics8020046
Chand S, Karuso P (2017) Isolation and total synthesis of two novel metabolites from the fissurellid mollusc Scutus antipodes. Tetrahedron Lett 58(10):1020–1023. https://doi.org/10.1016/j.tetlet.2017.01.096
Chaves Lopes F, Rosa de Castro M, Caldas Barbosa S, Primel EG, de Martinez Gaspar Martins C (2020) Effect of the UV filter, benzophenone-3, on biomarkers of the yellow clam (Amarilladesma mactroides) under different pH conditions. Mar Pollut Bull 158:111401. https://doi.org/10.1016/j.marpolbul.2020.111401
Chen X, Si L, Liu D, Proksch P, Zhang L, Zhou D, Lin W (2015a) Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur J Med Chem 93:182–195. https://doi.org/10.1016/j.ejmech.2015.02.006
Chen Y, Lin H, Li Z, Mou Q (2015b) The anti-allergic activity of polyphenol extracted from five marine algae. J Ocean Univ China 14(4):681–684. https://doi.org/10.1007/s11802-015-2601-5
Chrapusta E, Kaminski A, Duchnik K, Bober B, Adamski M, Bialczyk J (2017) Mycosporine-like amino acids: potential health and beauty ingredients. Mar Drugs 15(10). https://doi.org/10.3390/md15100326
Clish CB (2015) Metabolomics: an emerging but powerful tool for precision medicine. Cold Spring Harbor molecular case studies 1(1):a000588-a000588. https://doi.org/10.1101/mcs.a000588
Cockell CS, Knowland J (1999) Ultraviolet radiation screening compounds. Biol Rev 74(3):311–345. https://doi.org/10.1111/j.1469-185X.1999.tb00189.x
Conceição K, de Cena GL, da Silva VA, de Oliveira Neto XA, de Andrade VM, Tada DB, Richardson M, de Andrade SA, Dias SA, Castanho MARB, Lopes-Ferreira M (2020) Design of bioactive peptides derived from CART sequence isolated from the toadfish Thalassophryne nattereri. 3 Biotech 10(4):162. https://doi.org/10.1007/s13205-020-2151-4
D'Agostino PM, Woodhouse JN, Liew HT, Sehnal L, Pickford R, Wong HL, Burns BP, Neilan BA (2019) Bioinformatic, phylogenetic and chemical analysis of the UV-absorbing compounds scytonemin and mycosporine-like amino acids from the microbial mat communities of Shark Bay, Australia. Environ Microbiol 21(2):702–715. https://doi.org/10.1111/1462-2920.14517
De La Coba F, Aguilera J, Figueroa FL, De Gálvez MV, Herrera E (2009) Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J Appl Phycol 21(2):161–169. https://doi.org/10.1007/s10811-008-9345-1
Dewapriya P, Kim S-k (2014) Marine microorganisms: an emerging avenue in modern nutraceuticals and functional foods. Food Res Int 56:115–125. https://doi.org/10.1016/j.foodres.2013.12.022
Domínguez-Pérez D, Campos A, Alexei Rodríguez A, Turkina MV, Ribeiro T, Osorio H, Vasconcelos V, Antunes A (2018) Proteomic analyses of the unexplored sea anemone Bunodactis verrucosa. Mar Drugs 16(2):42. https://doi.org/10.3390/md16020042
Dunlap WC, Chalker BE (1986) Identification and quantitation of near-UV absorbing compounds (S-320) in a hermatypic scleractinian. Coral Reefs 5(3):155–159. https://doi.org/10.1007/BF00298182
Dunlap WC, Yamamoto Y (1995) Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp Biochem Physiol B Biochem 112(1):105–114. https://doi.org/10.1016/0305-0491(95)00086-N
Figueroa FL (2021) Mycosporine-like amino acids from marine resource. Mar Drugs 19(1). https://doi.org/10.3390/md19010018
Figueroa FL, Bonomi Barufi J, Malta EJ, Conde-Álvarez R, Nitschke U, Arenas F, Mata M, Connan S, Abreu MH, Marquardt R, Vaz-Pinto F, Konotchick T, Celis-Plá PS, Hermoso M, Ordoñez G, Ruiz E, Flores P, De Los RJ, Kirke D, Chow F, Nassar CA, Robledo D, Pérez-Ruzafa Á, Bañares-España E, Altamirano M, Jiménez C, Korbee N, Bischof K, Stengel DB (2014) Short-term effects of increasing CO2, nitrate and temperature on three mediterranean macroalgae: biochemical composition. Aquat Biol 22:177–193. https://doi.org/10.3354/ab00610
Fry BG, Roelants K, Winter K, Hodgson WC, Griesman L, Kwok HF, Scanlon D, Karas J, Shaw C, Wong L, Norman JA (2010) Novel venom proteins produced by differential domain-expression strategies in beaded lizards and gila monsters (genus Heloderma). Mol Biol Evol 27(2):395–407. https://doi.org/10.1093/molbev/msp251
Fuentes-Tristan S, Parra-Saldivar R, Iqbal HMN, Carrillo-Nieves D (2019) Bioinspired biomolecules: mycosporine-like amino acids and scytonemin from Lyngbya sp with UV-protection potentialities. J Photochem Photobiol B Biol:201. https://doi.org/10.1016/j.jphotobiol.2019.111684
Gao Q, Garcia-Pichel F (2011) An ATP-Grasp ligase involved in the last biosynthetic step of the iminomycosporine shinorine in Nostoc punctiforme ATCC 29133. J Bacteriol 193(21):5923–5928. https://doi.org/10.1128/JB.05730-11
García PE, Diéguez MC, Ferraro MA, Zagarese HE, Pérez AP (2010) Mycosporine-like amino acids in freshwater copepods: potential sources and some factors that affect their bioaccumulation. Photochem Photobiol 86(2):353–359. https://doi.org/10.1111/j.1751-1097.2009.00670.x
Garcia-Pichel F, Wingard CE, Castenholz RW (1993) Evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl Environ Microbiol 59(1):170–176 https://www.scopus.com/inward/record.uri?eid=2-s2.0-0027507368&partnerID=40&md5=19f7f086ace05448541dba66b359add9
Gaspar LR, Maia Campos PMBG (2006) Evaluation of the photostability of different UV filter combinations in a sunscreen. Int J Pharm 307(2):123–128. https://doi.org/10.1016/j.ijpharm.2005.08.029
Gaspar LR, Camargo FB, Gianeti MD, Maia Campos PMBG (2008) Evaluation of dermatological effects of cosmetic formulations containing Saccharomyces cerevisiae extract and vitamins. Food Chem Toxicol 46(11):3493–3500. https://doi.org/10.1016/j.fct.2008.08.028
Geraldes V, Pinto E (2021) Mycosporine-like amino acids (MAAs): biology, chemistry and identification features. Pharmaceuticals 14(1). https://doi.org/10.3390/ph14010063
Greenspoon J, Ahluwalia R, Juma N, Rosen CF (2013) Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis 24(1):29–32. https://doi.org/10.1097/DER.0b013e31827edc8b
Gröniger A, Sinha RP, Klisch M, Häder DP (2000) Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae - a database. J Photochem Photobiol B Biol 58(2-3):115–122. https://doi.org/10.1016/S1011-1344(00)00112-3
Harms H, Klöckner A, Schrör J, Josten M, Kehraus S, Crüsemann M, Hanke W, Schneider T, Schäberle TF, König GM (2018) Antimicrobial dialkylresorcins from marine-derived microorganisms: insights into their mode of action and putative ecological relevance. Planta Med 84(18):1363–1371. https://doi.org/10.1055/a-0653-7451
Hernando M, Carreto JI, Carignan MO, Ferreyra GA, Gross C (2002) Effects of solar radiation on growth and mycosporine-like amino acids content in Thalassiosira sp, an Antarctic diatom. Polar Biol 25(1):12–20. https://doi.org/10.1007/s003000100306
Hong L-L, Yu H-B, Wang J, Jiao W-H, Cheng B-H, Yang F, Zhou Y-J, Gu B-B, Song S-J, Lin H-W (2017) Unusual anti-allergic diterpenoids from the marine sponge Hippospongia lachne. Sci Rep 7(1):43138. https://doi.org/10.1038/srep43138
Huwaidi A, Ahmad KA, Magdy M, Shaharuddin NA, Ikeno S, Syahir A (2020) Identification of mycosporine-like amino acids and expression of 3-dehydroquinate synthase gene in UV radiations-induced Deinococcus radiodurans R1. Malays J Biochem Mol Biol 23(2):19–29 https://www.scopus.com/inward/record.uri?eid=2-s2.0-85094955163&partnerID=40&md5=6ec5294960110f4791d38868fdbe86de
Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T (2003) UV-induced skin damage. Toxicol 189(1-2):21–39. https://doi.org/10.1016/s0300-483x(03)00150-1
Ingebrigtsen RA, Hansen E, Andersen JH, Eilertsen HC (2016) Light and temperature effects on bioactivity in diatoms. J Appl Phycol 28:939–950. https://doi.org/10.1007/s10811-015-0631-4
Iwasaki A, Fujimura H, Okamoto S, Kudo T, Hoshina S, Sumimoto S, Teruya T, Suenaga K (2018) Isolation of jahanene and jahanane, and total synthesis of the jahanyne family. J Org Chem 83(17):9592–9603. https://doi.org/10.1021/acs.joc.8b00310
Jang Y, Shin H, Lee MK, Kwon OS, Shin JS, Y-i K, Kim CW, Lee H-R, Kim M (2021) Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci Rep 11(1):821. https://doi.org/10.1038/s41598-020-80896-9
Ji X, Guo J, Liu Y, Lu A, Wang Z, Li Y, Yang S, Wang Q (2018) Marine-natural-product development: first discovery of nortopsentin alkaloids as novel antiviral, anti-phytopathogenic-fungus, and insecticidal agents. J Agric Food Chem 66(16):4062–4072. https://doi.org/10.1021/acs.jafc.8b00507
Jiao W-H, Hong L-L, Sun J-B, Piao S-J, Chen G-D, Deng H, Wang S-P, Yang F, Lin H-W (2017) (±)-Hippolide J – a pair of unusual antifungal enantiomeric sesterterpenoids from the marine sponge Hippospongia lachne. Eur J Org Chem 2017(24):3421–3426. https://doi.org/10.1002/ejoc.201700248
Jiménez C (2018) Marine natural products in medicinal chemistry. ACS Med Chem Lett 9(10):959–961. https://doi.org/10.1021/acsmedchemlett.8b00368
Kageyama H, Waditee-Sirisattha R (2019) Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: molecular and cellular mechanisms in the protection of skin-aging. Mar Drugs 17(4). https://doi.org/10.3390/md17040222
Kawakami CM, Gaspar LR (2015) Mangiferin and naringenin affect the photostability and phototoxicity of sunscreens containing avobenzone. J Photochem Photobiol B Biol 151:239–247. https://doi.org/10.1016/j.jphotobiol.2015.08.014
Kim GJ, Li X, Kim SH, Yang I, Hahn D, Chin J, Nam SJ, Nam JW, Nam DH, Oh DC, Chang HW, Choi H (2018a) Seongsanamides A-D: antiallergic bicyclic peptides from Bacillus safensis KCTC 12796BP. Org Lett 20(23):7539–7543. https://doi.org/10.1021/acs.orglett.8b03293
Kim MC, Machado H, Jang KH, Trzoss L, Jensen PR, Fenical W (2018b) Integration of genomic data with NMR analysis enables assignment of the full stereostructure of neaumycin B, a potent inhibitor of glioblastoma from a marine-derived micromonospora. J Am Chem Soc 140(34):10775–10784. https://doi.org/10.1021/jacs.8b04848
Koltover VK (2010) Antioxidant biomedicine: from free radical chemistry to systems biology mechanisms. Russ Chem Bull 59(1):37–42. https://doi.org/10.1007/s11172-010-0042-2
Korbee N, Figueroa FL, Aguilera J (2006) Accumulation of mycosporine-like amino acids (MAAs): biosynthesis, photocontrol and ecophysiological functions. Rev Chil Hist Nat 79(1):119–132 https://www.scopus.com/inward/record.uri?eid=2-s2.0-33646812594&partnerID=40&md5=0fd7153aaff318668053fec73019fa29
Kuehnbaum NL, Britz-McKibbin P (2013) New advances in separation science for metabolomics: resolving chemical diversity in a post-genomic era. Chem Rev 113(4):2437–2468. https://doi.org/10.1021/cr300484s
Kwan JC, Donia MS, Han AW, Hirose E, Haygood MG, Schmidt EW (2012) Genome streamlining and chemical defense in a coral reef symbiosis. Proc Natl Acad Sci U S A 109(50):20655–20660. https://doi.org/10.1073/pnas.1213820109
Lai KH, You WJ, Lin CC, El-Shazly M, Liao ZJ, Su JH (2017) Anti-inflammatory dembranoids from the soft coral Lobophytum crassum. Mar Drugs 15(10). https://doi.org/10.3390/md15100327
Lauritano CIA (2018) Grand challenges in marine biotechnology: overview of recent EU-funded projects. In: TA Rampelotto P. (ed) Grand challenges in marine biotechnology. Grand challenges in biology and biotechnology. Springer
Lauritano C, Andersen JH, Hansen E, Albrigtsen M, Escalera L, Esposito F, Helland K, Hanssen KØ, Romano G, Ianora A (2016a) Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front Mar Sci 3(68). https://doi.org/10.3389/fmars.2016.00068
Lauritano C, Martín J, de la Cruz M, Reyes F, Romano G, Ianora A (2018) First identification of marine diatoms with anti-tuberculosis activity. Sci Rep 8(1):2284. https://doi.org/10.1038/s41598-018-20611-x
Lauritano C, Ferrante MI, Rogato A (2019) Marine natural products from microalgae: an -omics overview. Mar Drugs 17(5). https://doi.org/10.3390/md17050269
Lindström J, Grebner W, Rigby K, Selander E (2017) Effects of predator lipids on dinoflagellate defence mechanisms - increased bioluminescence capacity. Sci Rep 7(1):13104. https://doi.org/10.1038/s41598-017-13293-4
Liu Q-M, Xie C-L, Gao Y-Y, Liu B, Lin W-X, Liu H, Cao M-J, Su W-J, Yang X-W, Liu G-M (2018) Deep-sea-derived butyrolactone I suppresses ovalbumin-induced anaphylaxis by regulating mast cell function in a murine model. J Agric Food Chem 66(22):5581–5592. https://doi.org/10.1021/acs.jafc.8b01674
Llewellyn CA, Airs RL (2010) Distribution and abundance of MAAs in 33 species of microalgae across 13 classes. Mar Drugs 8(4):1273–1291. https://doi.org/10.3390/md8041273
Llewellyn CA, Greig C, Silkina A, Kultschar B, Hitchings MD, Farnham G (2020) Mycosporine-like amino acid and aromatic amino acid transcriptome response to UV and far-red light in the cyanobacterium Chlorogloeopsis fritschii PCC 6912. Sci Rep 10(1):20638. https://doi.org/10.1038/s41598-020-77402-6
Lohr KE, Khattri RB, Guingab-Cagmat J, Camp EF, Merritt ME, Garrett TJ, Patterson JT (2019) Metabolomic profiles differ among unique genotypes of a threatened Caribbean coral. Sci Rep 9(1):6067. https://doi.org/10.1038/s41598-019-42434-0
Lyu C, Chen T, Qiang B, Liu N, Wang H, Zhang L, Liu Z (2020) CMNPD: a comprehensive marine natural products database towards facilitating drug discovery from the ocean. Nucleic Acids Res. https://doi.org/10.1093/nar/gkaa763
Ma X, Li L, Zhu T, Ba M, Li G, Gu Q, Guo Y, Li D (2013) Phenylspirodrimanes with anti-HIV activity from the sponge-derived fungus Stachybotrys chartarum MXH-X73. J Nat Prod 76(12):2298–2306. https://doi.org/10.1021/np400683h
Marggraf MB, Panteleev PV, Emelianova AA, Sorokin MI, Bolosov IA, Buzdin AA, Kuzmin DV, Ovchinnikova TV (2018) Cytotoxic potential of the novel horseshoe crab peptide Polyphemusin III. Mar Drugs 16(12):466 https://www.mdpi.com/1660-3397/16/12/466
Martin HL, Smith L, Tomlinson DC (2014) Multidrug-resistant breast cancer: current perspectives. Breast Cancer (Dove Med Press) 6:1–13. https://doi.org/10.2147/bctt.S37638
Mei C, Zhou S, Zhu L, Ming J, Zeng F, Xu R (2017) Antitumor effects of Laminaria extract fucoxanthin on lung cancer. Mar Drugs 15(2). https://doi.org/10.3390/md15020039
Mert Ozupek N, Cavas L (2017) Triterpene glycosides associated antifouling activity from Holothuria tubulosa and H. polii. Reg Stud Mar Sci 13:32–41. https://doi.org/10.1016/j.rsma.2017.04.003
Miyamoto KT, Komatsu M, Ikeda H (2014) Discovery of gene cluster for mycosporine-like amino acid biosynthesis from Actinomycetales microorganisms and production of a novel mycosporine-like amino acid by heterologous expression. Appl Environ Microbiol 80(16):5028–5036. https://doi.org/10.1128/AEM.00727-14
Mohamed TA, Elshamy AI, Hussien TA, Su J-H, Sheu J-H, Hegazy MEF (2017) Lobophylins F-H: three new cembrene diterpenoids from soft coral Lobophytum crassum. J Asian Nat Prod Res 19(3):201–207. https://doi.org/10.1080/10286020.2016.1196673
Nakajima S, Watashi K, Ohashi H, Kamisuki S, Izaguirre-Carbonell J, Kwon AT, Suzuki H, Kataoka M, Tsukuda S, Okada M, Moi ML, Takeuchi T, Arita M, Suzuki R, Aizaki H, Kato T, Suzuki T, Hasegawa H, Takasaki T, Sugawara F, Wakita T (2016) Fungus-derived neoechinulin B as a novel antagonist of liver X receptor, identified by chemical genetics using a hepatitis C virus cell culture system. J Virol 90(20):9058–9074. https://doi.org/10.1128/jvi.00856-16
Napolitano A, Panzella L, Monfrecola G, d'Ischia M (2014) Pheomelanin-induced oxidative stress: bright and dark chemistry bridging red hair phenotype and melanoma. Pigment Cell Melanoma Res 27(5):721–733. https://doi.org/10.1111/pcmr.12262
Oren A, Gunde-Cimerman N (2007) Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett 269(1):1–10. https://doi.org/10.1111/j.1574-6968.2007.00650.x
Orencia MC, Yoon JS, Ness JE, Stemmer WPC, Stevens RC (2001) Predicting the emergence of antibiotic resistance by directed evolution and structural analysis. Nat Struct Biol 8(3):238–242. https://doi.org/10.1038/84981
Orfanoudaki M, Hartmann A, Kamiya M, West J, Ganzera M (2020) Chemotaxonomic study of Bostrychia spp. (Ceramiales, Rhodophyta) based on their mycosporine-like amino acid content. Molecules 25(14). https://doi.org/10.3390/molecules25143273
Pailee P, Mahidol C, Ruchirawat S, Prachyawarakorn V (2017) Sterols from Thai marine sponge Petrosia (Strongylophora) sp. and their cytotoxicity. Mar Drugs 15(3). https://doi.org/10.3390/md15030054
Parailloux M, Godin S, Fernandes SCM, Lobinski R (2020) Untargeted analysis for mycosporines and mycosporine-like amino acids by hydrophilic interaction liquid chromatography (HILIC)—electrospray orbitrap MS2/MS3. Antioxidants 9(1185).
Parailloux M, Godin S, Fernandes SCM, Lobinski R (2020a) Untargeted analysis for mycosporines and mycosporine-like amino acids by hydrophilic interaction liquid chromatography (HILIC)—electrospray orbitrap MS2/MS3. Antioxidants 9(12):1–26. https://doi.org/10.3390/antiox9121185
Parailloux MGS, Fernandes SCM, Lobinski R (2020b) Untargeted analysis for mycosporines and mycosporine-like amino acids by hydrophilic interaction liquid chromatography (HILIC)—electrospray orbitrap MS2/MS3. Antioxidants 9(1185)
Parish T (2019) Steps to address anti-microbial drug resistance in today’s drug discovery. Expert Opin Drug Discovery 14(2):91–94. https://doi.org/10.1080/17460441.2019.1550481
Pereira F (2019) Have marine natural product drug discovery efforts been productive and how can we improve their efficiency? Expert Opin Drug Discovery 14(8):717–722. https://doi.org/10.1080/17460441.2019.1604675
Pereira F, Aires-de-Sousa J (2018) Computational methodologies in the exploration of marine natural product leads. Mar Drugs 16(7). https://doi.org/10.3390/md16070236
Pope MA, Spence E, Seralvo V, Gacesa R, Heidelberger S, Weston AJ, Dunlap WC, Shick JM, Long PF (2015) O-Methyltransferase is shared between the pentose phosphate and shikimate pathways and is essential for mycosporine-like amino acid biosynthesis in Anabaena variabilis ATCC 29413. ChemBioChem 16(2):320–327. https://doi.org/10.1002/cbic.201402516
Portwich A, Garcia-Pichel F (1999) Ultraviolet and osmotic stresses induce and regulate the synthesis of mycosporines in the cyanobacterium Chlorogloeopsis PCC 6912. Arch Microbiol 172(4):187–192. https://doi.org/10.1007/s002030050759
Premi S, Wallisch S, Mano CM, Weiner AB, Bacchiocchi A, Wakamatsu K, Bechara EJ, Halaban R, Douki T, Brash DE (2015) Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Sci 347(6224):842–847. https://doi.org/10.1126/science.1256022
Rajneesh SSP, Pathak J, Sinha RP (2017) Cyanobacterial factories for the production of green energy and value-added products: an integrated approach for economic viability. Renew Sust Energ Rev 69:578–595. https://doi.org/10.1016/j.rser.2016.11.110
Rastogi RP, Richa SRP, Singh SP, Häder DP (2010) Photoprotective compounds from marine organisms. J Ind Microbiol Biotechnol 37(6):537–558. https://doi.org/10.1007/s10295-010-0718-5
Rastogi RP, Sonani RR, Madamwar D, Incharoensakdi A (2016) Characterization and antioxidant functions of mycosporine-like amino acids in the cyanobacterium Nostoc sp. R76DM. Algal Res 16:110–118. https://doi.org/10.1016/j.algal.2016.03.009
Řezanka T, Temina M, Tolstikov AG, Dembitsky VM (2004) Natural microbial UV radiation filters — mycosporine-like amino acids. Folia Microbiol 49(4):339–352. https://doi.org/10.1007/BF03354663
Richa, Sinha RP (2013) Biomedical applications of mycosporine-like amino acids. In: Marine Microbiology: Bioactive Compounds and Biotechnological Applications (pp. 509-534). 10.1002/9783527665259.ch27
Rodríguez AA, Ständker L, Zaharenko AJ, Garateix AG, Forssmann WG, Béress L, Valdés O, Hernández Y, Laguna A (2012) Combining multidimensional liquid chromatography and MALDI-TOF-MS for the fingerprint analysis of secreted peptides from the unexplored sea anemone species Phymanthus crucifer. J Chromatogr B Anal Technol Biomed Life Sci 903:30–39. https://doi.org/10.1016/j.jchromb.2012.06.034
Rojas J, Londoño C, Ciro Y (2016) The health benefits of natural skin uva photo-protective compounds found in botanical sources. Int J Pharm Pharm Sci 8(3):13–23 https://www.scopus.com/inward/record.uri?eid=2-s2.0-84960083029&partnerID=40&md5=19df213f92203159074b9ebe549947f8
Rosic NN (2009) Versatile capacity of shuffled cytochrome P450s for dye production. Appl Microbiol Biotechnol 82(2):203–210. https://doi.org/10.1007/s00253-008-1812-8
Rosic NN (2012) Phylogenetic analysis of genes involved in mycosporine-like amino acid biosynthesis in symbiotic dinoflagellates. Appl Microbiol Biotechnol 94(1):29–37. https://doi.org/10.1007/s00253-012-3925-3
Rosic NN (2013) DNA shuffling of cytochromes P450 for indigoid pigment production. Methods Mol Biol 987:205–224. https://doi.org/10.1007/978-1-62703-321-3_18
Rosic NN (2019) Mycosporine-like amino acids: making the foundation for organic personalised sunscreens. Mar Drugs 17(11). https://doi.org/10.3390/md17110638
Rosic NN, Dove S (2011) Mycosporine-like amino acids from coral dinoflagellates. Appl Environ Microbiol 77(24):8478–8486. https://doi.org/10.1128/AEM.05870-11
Rosic NN, Braun C, Kvaskoff D (2015) Extraction and analysis of mycosporine-like amino acids in marine algae. Methods Mol Biol 1308:119–129. https://doi.org/10.1007/978-1-4939-2684-8_6
Salazar G, Paoli L, Alberti A, Huerta-Cepas J, Ruscheweyh H-J, Cuenca M, Field CM, Coelho LP, Cruaud C, Engelen S, Gregory AC, Labadie K, Marec C, Pelletier E, Royo-Llonch M, Roux S, Sánchez P, Uehara H, Zayed AA, Zeller G, Carmichael M, Dimier C, Ferland J, Kandels S, Picheral M, Pisarev S, Poulain J, Acinas SG, Babin M, Bork P, Boss E, Bowler C, Cochrane G, de Vargas C, Follows M, Gorsky G, Grimsley N, Guidi L, Hingamp P, Iudicone D, Jaillon O, Kandels-Lewis S, Karp-Boss L, Karsenti E, Not F, Ogata H, Pesant S, Poulton N, Raes J, Sardet C, Speich S, Stemmann L, Sullivan MB, Sunagawa S, Wincker P, Acinas SG, Babin M, Bork P, Bowler C, de Vargas C, Guidi L, Hingamp P, Iudicone D, Karp-Boss L, Karsenti E, Ogata H, Pesant S, Speich S, Sullivan MB, Wincker P, Sunagawa S (2019) Gene expression changes and community turnover differentially shape the global ocean metatranscriptome. Cell 179(5):1068–1083.e1021. https://doi.org/10.1016/j.cell.2019.10.014
Salim EI, Harras SF, Abdalla AG, Mona MH (2018) Syphacia muris infection in rats attenuates colorectal carcinogenesis through oxidative stress and gene expression alterations. Implications for modulatory effects by Bryostatin-1. Acta Parasitol 63(1):198–209. https://doi.org/10.1515/ap-2018-0023
Sánchez-Quiles D, Blasco J, Tovar-Sánchez A (2020) Sunscreen components are a new environ concern in coastal waters: an overview. In: Tovar-Sánchez A, Sánchez-Quiles D, Blasco J (eds) Sunscreens in coastal ecosystems: occurrence, behavior, effect and risk. Springer International Publishing, pp 1–14. https://doi.org/10.1007/698_2019_439
Schofield MM, Jain S, Porat D, Dick GJ, Sherman DH (2015) Identification and analysis of the bacterial endosymbiont specialized for production of the chemotherapeutic natural product ET-743. Environ Microbiol 17(10):3964–3975. https://doi.org/10.1111/1462-2920.12908
Sharifan H, Klein D, Morse AN (2016) UV filters are an environmental threat in the Gulf of Mexico: a case study of Texas coastal zones. Oceanologia 58(4):327–335. https://doi.org/10.1016/j.oceano.2016.07.002
Sicherer SH (2011) Epidemiology of food allergy. J Allergy Clin Immunol 127. https://doi.org/10.1016/j.jaci.2010.11.044
Singh SP, Klisch M, Sinha RP, Häder DP (2008a) Effects of abiotic stressors on synthesis of the mycosporine-like amino acid shinorine in the cyanobacterium Anabaena variabilis PCC 7937. PhotoChem Photobiol 84(6):1500–1505. https://doi.org/10.1111/j.1751-1097.2008.00376.x
Singh SP, Kumari S, Rastogi RP, Singh KL, Sinha RP (2008b) Mycosporine-like amino acids (MAAs): chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J Exp Biol 46(1):7–17
Singh SK, Kaur R, Bansal A, Kapur S, Sundaram S (2019) Biotechnological exploitation of cyanobacteria and microalgae for bioactive compounds. In Biotechnological Production of Bioactive Compounds (pp. 221-259). https://doi.org/10.1016/B978-0-444-64323-0.00008-4
Sinha RP, Klisch M, Gröniger A, Häder DP (1998) Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J Photochem Photobiol B Biol 47(2-3):83–94. https://doi.org/10.1016/S1011-1344(98)00198-5
Sinha RP, Ambasht NK, Sinha JP, Klisch M, Häder DP (2003) UV-B-induced synthesis of mycosporine-like amino acids in three strains of Nodularia (cyanobacteria). J Photochem Photobiol B Biol 71(1-3):51–58. https://doi.org/10.1016/j.jphotobiol.2003.07.003
Sinha RP, Singh SP, Häder DP (2007) Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J Photochem Photobiol B Biol 89(1):29–35. https://doi.org/10.1016/j.jphotobiol.2007.07.006
Sperlich J, Teusch N (2018) Pseudopterosin inhibits proliferation and 3D invasion in triple-negative breast cancer by agonizing glucocorticoid receptor alpha. Molecules 23(8). https://doi.org/10.3390/molecules23081992
Suh SS, Hwang J, Park M, Seo HH, Kim HS, Lee JH, Moh SH, Lee TK (2014) Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar Drugs 12(10):5174–5187. https://doi.org/10.3390/md12105174
Tarazona G, Santamaría G, Cruz PG, Fernández R, Pérez M, Martínez-Leal JF, Rodríguez J, Jiménez C, Cuevas C (2017) Cytotoxic anomoian B and aplyzanzine B, new bromotyrosine alkaloids from Indonesian sponges. ACS Omega 2(7):3494–3501. https://doi.org/10.1021/acsomega.7b00417
Tartarotti B, Sommaruga R (2006) Seasonal and ontogenetic changes of mycosporine-like amino acids in planktonic organisms from an alpine lake. Limnol Oceanogr 51(3):1530–1541. https://doi.org/10.4319/lo.2006.51.3.1530
Tetko IV, Engkvist O (2020) From big data to artificial intelligence: chemoinformatics meets new challenges. J Cheminform 12(1):74. https://doi.org/10.1186/s13321-020-00475-y
Trager MH, Geskin LJ, Samie FH, Liu L (2020) Biomarkers in melanoma and non-melanoma skin cancer prevention and risk stratification. Exp Dermatol. https://doi.org/10.1111/exd.14114
Turner PJ, Campbell DE, Motosue MS, Campbell RL (2020) Global trends in anaphylaxis epidemiology and clinical implications. J Allergy Clinic Immunol: In Practice 8(4):1169-1176. https://doi.org/10.1016/j.jaip.2019.11.027
Uzair B, Menaa F, Khan BA, Mohammad FV, Ahmad VU, Djeribi R, Menaa B (2018) Isolation, purification, structural elucidation and antimicrobial activities of kocumarin, a novel antibiotic isolated from actinobacterium Kocuria marina CMG S2 associated with the brown seaweed Pelvetia canaliculata. Microbiol Res 206:186–197. https://doi.org/10.1016/j.micres.2017.10.007
Vo TS, Ngo DH, Kim SK (2012) Potential targets for anti-inflammatory and anti-allergic activities of marine algae: an overview. Inflamm Allergy Drug Targets 11(2):90–101. https://doi.org/10.2174/187152812800392797
Voolstra C, Miller D, Ragan M, Hoffmann A, Hoegh-Guldberg O, Bourne D, Ball E, Ying H, Foret S, Takahashi S, Weynberg K, van Oppen M, Morrow K, Chan CX, Rosic N, Leggat W, Sprungala S, Imelfort M, Tyson G, Kassahn K, Lundgren P, Beeden R, Ravasi T, Berumen M, Abel E, Fyffe T (2015) The ReFuGe 2020 Consortium—using “omics” approaches to explore the adaptability and resilience of coral holobionts to environmental change. Front Mar Sci 2(68). https://doi.org/10.3389/fmars.2015.00068
Waditee-Sirisattha R, Kageyama H, Sopun W, Tanaka Y, Takabe T (2014) Identification and upregulation of biosynthetic genes required for accumulation of mycosporine-2-glycine under salt stress conditions in the halotolerant cyanobacterium Aphanothece halophytica. Appl Environ Microbiol 80(5):1763–1769. https://doi.org/10.1128/AEM.03729-13
Waditee-Sirisattha R, Kageyama H, Fukaya M, Rai V, Takabe T (2015) Nitrate and amino acid availability affects glycine betaine and mycosporine-2-glycine in response to changes of salinity in a halotolerant cyanobacterium Aphanothece halophytica. FEMS Microbiol Lett 362(23). https://doi.org/10.1093/femsle/fnv198
Wang J, Chen F, Liu Y, Liu Y, Li K, Yang X, Liu S, Zhou X, Yang J (2018) Spirostaphylotrichin X from a marine-derived fungus as an anti-influenza agent targeting RNA polymerase PB2. J Nat Prod 81(12):2722–2730. https://doi.org/10.1021/acs.jnatprod.8b00656
Watanabe S, Sudo K, Nagashima T, Takemura T, Kawase H, Nozawa T (2011) Future projections of surface UV-B in a changing climate. Journal of Geophysical Research: Atmospheres 116(D16). https://doi.org/10.1029/2011JD015749
WCRF (2018) Breast cancer statistics. https://www.wcrf.org/dietandcancer/cancer-trends/breast-cancer-statistics
White AM, Dao K, Vrubliauskas D, Könst ZA, Pierens GK, Mándi A, Andrews KT, Skinner-Adams TS, Clarke ME, Narbutas PT, Sim DC, Cheney KL, Kurtán T, Garson MJ, Vanderwal CD (2017) Catalyst-controlled stereoselective synthesis secures the structure of the antimalarial isocyanoterpene pustulosaisonitrile-1. J Organomet Chem 82(24):13313–13323. https://doi.org/10.1021/acs.joc.7b02421
Whittock AL, Turner MAP, Coxon DJL, Woolley JM, Horbury MD, Stavros VG (2020) Reinvestigating the photoprotection properties of a mycosporine amino acid motif. Front Chem 8. https://doi.org/10.3389/fchem.2020.574038
WHO (2018a) Antimicrobial resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
WHO (2018b) Cancer - World Health Organization. https://www.who.int/news-room/fact-sheets/detail/cancer
Wood L (2020) Global allergy treatment market - growth, trends & forecast to 2025
Xie C-L, Liu Q, Xia J-M, Gao Y, Yang Q, Shao Z-Z, Liu G, Yang X-W (2017) Anti-allergic compounds from the deep-sea-derived actinomycete Nesterenkonia flava MCCC 1 K00610. Mar Drugs 15(3). https://doi.org/10.3390/md15030071
Yakovleva IM, Baird AH (2005) Ontogenetic change in the abundance of mycosporine-like amino acids in non-zooxanthellate coral larvae. Coral Reefs 24(3):443–452. https://doi.org/10.1007/s00338-005-0005-5
Yamashita A, Tamaki M, Kasai H, Tanaka T, Otoguro T, Ryo A, Maekawa S, Enomoto N, de Voogd NJ, Tanaka J, Moriishi K (2017) Inhibitory effects of metachromin A on hepatitis B virus production via impairment of the viral promoter activity. Antivir Res 145:136–145. https://doi.org/10.1016/j.antiviral.2017.08.001
Yao X, Cao D, Wang F, Zhang W, Ma C, Song M (2019) An overview of omics approaches to characterize the effect of perfluoroalkyl substances in environmental health. TrAC Trends Anal Chem 121:115367. https://doi.org/10.1016/j.trac.2018.12.021
Yuan YV, Westcott ND, Hu C, Kitts DD (2009) Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem 112(2):321–328. https://doi.org/10.1016/j.foodchem.2008.05.066
Acknowledgements
The author would like to thank the anonymous reviewers, as well as Dr Jacinta Arellano and Ms Isidora Skrlin, for their critical reviews of this paper.
Author information
Authors and Affiliations
Contributions
NR wrote this mini-review.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rosic, N.N. Recent advances in the discovery of novel marine natural products and mycosporine-like amino acid UV-absorbing compounds. Appl Microbiol Biotechnol 105, 7053–7067 (2021). https://doi.org/10.1007/s00253-021-11467-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11467-9