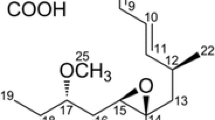
Abstract
The telomerase activator cycloastragenol (CA) is regarded as a potential anti-aging drug with promising applications in the food and medical industry. However, one remaining challenge is the low efficiency of CA production. Herein, we developed an enzyme-based approach by applying two enzymes (β-xylosidase: Xyl-T; β-glucosidase: Bgcm) for efficient CA production. Both key glycosidases, mined by activity tracking or homology sequence screening, were successfully over-expressed and showed prominent enzymatic activity profiles, including widely pH stability (Xyl-T: pH 3.0–8.0; Bgcm: pH 4.0–10.0), high catalytic efficiency (kcat/Km: 0.096 mM-1s−1 (Xyl-T) and 3.08 mM-1s−1 (Bgcm)), and mesophilic optimum catalytic temperature (50 °C). Besides, the putative catalytic residues (Xyl-T: Asp311/Glu 521; Bgcm: Asp311/Glu 521) and the potential substrate-binding mechanism of Xyl-T and Bgcm were predicted by comprehensive computational analysis, providing valuable insight into the hydrolysis of substrates at the molecular level. Notably, a rationally designed two-step reaction process was introduced to improve the CA yield and increased up to 96.5% in the gram-scale production, providing a potential alternative for the industrial CA bio-production. In essence, the explored enzymes, the developed enzyme-based approach, and the obtained knowledge from catalytic mechanisms empower researchers to further engineer the CA production and might be applied for other chemicals synthesis.
Key points
• A β-xylosidase and a β-glucosidase were mined to hydrolyze ASI into CA.
• The two recombinant glycosidases showed prominent catalytic profiles.
• Two-step enzymatic catalysis for CA production from ASI was developed.

Graphical abstract







Similar content being viewed by others
References
Adari BR, Alavala S, George SA, Meshram HM, Tiwari AK, Sarma A (2016) Synthesis of rebaudioside-A by enzymatic transglycosylation of stevioside present in the leaves of Stevia rebaudiana Bertoni. Food Chem 200:154–158. https://doi.org/10.1016/j.foodchem.2016.01.033
Aharoni A (2009) Mining for new enzymes. Microb Biotechnol 2:128–129. https://doi.org/10.1111/j.1751-7915.2009.00090_1.x
Allen WJ, Balius TE, Mukherjee S, Brozell SR, Moustakas DT, Lang PT, Case DA, Kuntz ID, Rizzo RC (2015) DOCK 6: Impact of new features and current docking performance. J Comput Chem 36:1132–1156. https://doi.org/10.1002/jcc.23905
Bedir E, Kula C, Öner Ö, Altaş M, Tağ Ö, Öngen (2015) Microbial transformation of astragalus sapogenins using Cunninghamella blakesleeana NRRL 1369 and Glomerella fusarioides ATCC 9552. J Mol Catal B: Enzym 115:29–34. https://doi.org/10.1016/j.molcatb.2015.01.013
Chapman J, Ismail AE, Dinu CZ (2018) Industrial applications of enzymes: recent advances, techniques, and outlooks. Catalysts 8:238. https://doi.org/10.3390/catal8060238
Chen C-Y, Fu Y-J, Zu Y-G, Wang W, Mu F-S, Luo M, Li C-Y, Gu C-B, Zhao C-J (2013) Biotransformation of saponins to astragaloside IV from Radix Astragali by immobilized Aspergillus niger. Biocatal Agric Biotechnol 2:196–203. https://doi.org/10.1016/j.bcab.2013.03.007
Cheng L, Zhang H, Liang H, Sun X, Shen X, Wang J, Wang W, Yuan Q, Ri H-I, Kim T-M, Kang M-S, Linhardtd RJ (2020) Enzymatic bioconversion of cycloastragenol-6-O-β-D-glucoside into cycloastragenol by a novel recombinant β-glucosidase from Phycicoccus sp. Soil748. Process Biochem 90:81–88. https://doi.org/10.1016/j.procbio.2019.11.006
Cleland WW (1979) Statistical analysis of enzyme kinetic data. Methods Enzymol 63:103–138. https://doi.org/10.1016/0076-6879(79)63008-2
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2:1511–1519. https://doi.org/10.1002/pro.5560020916
Cui H, Stadtmüller TH, Jiang Q, Jaeger K-E, Schwaneberg U, Davari M (2020) How to engineer organic solvent resistant enzymes: insights from combined molecular dynamics and directed evolution study. ChemCatChem 12:1–12. https://doi.org/10.1002/cctc.202000422
De França Passos D, Pereira N Jr, de Castro AM (2018) A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Curr Opin Green Sust Chem 14:60–66. https://doi.org/10.1016/j.cogsc.2018.06.003
DeLano WL (2002) PyMol: an open-source molecular graphics tool. CCP4 Newsl Prot Crystallogr 40:82–92
Druzhinina IS, Kubicek CP (2017) Genetic engineering of Trichoderma reesei cellulases and their production. Microb Biotechnol 10:1485–1499. https://doi.org/10.1111/1751-7915.12726
Eisenberg D, Lüthy R, Bowie JU (1997) [20] VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol 277:396–404. https://doi.org/10.1016/S0076-6879(97)77022-8
Feng LM, Lin XH, Huang FX, Cao J, Qiao X, Guo DA, Ye M (2014) Smith degradation, an efficient method for the preparation of cycloastragenol from astragaloside IV. Fitoterapia 95:42–50. https://doi.org/10.1016/j.fitote.2014.02.014
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem 17:490–519. https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<490::AIDJCC1>3.0.CO;2-P
Hrmova M, De Gori R, Smith BJ, Vasella A, Varghese JN, Fincher GB (2004) Three-dimensional structure of the barley β-d-glucan glucohydrolase in complex with a transition state mimic. J Biol Chem 279:4970–4980. https://doi.org/10.1074/jbc.M307188200
Ishida N, Okubo A, Kawai H, Yamazaki S, Toda S (1980) Interaction of amino acids with transition metal ions in solution (I) solution structure of L-lysine with Co (II) and Cu (II) ions as studied by nuclear magnetic resonance spectroscopy. Agric Biol Chem 44:263–270. https://doi.org/10.1080/00021369.1980.10863934
Jeya M, Joo A-R, Lee K-M, Tiwari MK, Lee K-M, Kim S-H, Lee J-K (2010) Characterization of β-glucosidase from a strain of Penicillium purpurogenum KJS506. Appl Microbiol Biotechnol 86:1473–1484. https://doi.org/10.1007/s00253-009-2395-8
Katayama H, Nagasu T, Oda Y (2001) Improvement of in-gel digestion protocol for peptide mass fingerprinting by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 15:1416–1421. https://doi.org/10.1002/rcm.379
Kitagawa I, Wang H, Takagi A, Fuchida M, Miura I, Yoshikawa M (1983) Saponin and sapogenol. XXXIV. chemical constituents of astragali radix, the root of astragalus membranaceus bunge. (1). cycloastragenol, the 9, 19-cycloanostane-type aglycone of astragalosides, and the artifact aglycone astragenol. Chem Pharm Bull 31:689–697. https://doi.org/10.1248/cpb.31.689
Knob A, Terrasan CRF, Carmona EC (2010) β-xylosidases from filamentous fungi: an overview. W J Microbiol Biotechnol 26:389–407. https://doi.org/10.1007/s11274-009-0190-4
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291. https://doi.org/10.1107/S0021889892009944
Li L, Hou X, Xu R, Liu C, Tu M (2017) Research review on the pharmacological effects of astragaloside IV. Fundam Clin Pharmacol 31:17–36. https://doi.org/10.1111/fcp.12232
Li Q, Wu T, Qi Z, Zhao L, Pei J, Tang F (2018) Characterization of a novel thermostable and xylose-tolerant GH 39 β-xylosidase from Dictyoglomus thermophilum. BMC Biotechnol 18:29. https://doi.org/10.1186/s12896-018-0440-3
Li Q, Wu T, Zhao L, Pei J, Wang Z, Xiao W (2019) Highly efficient biotransformation of astragaloside IV to cycloastragenol by sugar-stimulated β-glucosidase and β-xylosidase from Dictyoglomus thermophilum. J Microbiol Biotechnol 29:1882–1893. https://doi.org/10.4014/jmb.1807.07020
Liu T, Liao JX, Hu Y, Tu YH, Sun JS (2017) Synthetic access toward cycloastragenol glycosides. J Org Chem 82:4170–4178. https://doi.org/10.1021/acs.joc.7b00080
Mahasenan KV, Batuecas MT, De Benedetti S, Kim C, Rana N, Lee M, Hesek D, Fisher JF, Sanz-Aparicio J, Hermoso JA (2019) Catalytic cycle of glycoside hydrolase BglX from Pseudomonas aeruginosa and its implications for biofilm formation. ACS Chem Biol 15:189–196. https://doi.org/10.1021/acschembio.9b00754
Mak WS, Tran S, Marcheschi R, Bertolani S, Thompson J, Baker D, Liao JC, Siegel JB (2015) Integrative genomic mining for enzyme function to enable engineering of a non-natural biosynthetic pathway. Nat Commun 6:1–10. https://doi.org/10.1038/ncomms10005
Margolles-Clark E, Tenkanen M, Nakari-Setälä T, Penttilä M (1996) Cloning of genes encoding alpha-L-arabinofuranosidase and beta-xylosidase from Trichoderma reesei by expression in Saccharomyces cerevisiae. Appl Environ Microbiol 62:3840–3846 http://aem.asm.org/content/62/10/3840
Molgora B, Bateman R, Sweeney G, Finger D, Dimler T, Effros RB, Valenzuela HF (2013) Functional assessment of pharmacological telomerase activators in human T cells. Cells 2:57–66. https://doi.org/10.3390/cells2010057
Park CS, Yoo MH, Noh KH, Oh DK (2010) Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol 87:9–19. https://doi.org/10.1007/s00253-010-2567-6
Paul ID, Bhole GP, Chaudhari JR (2014) A review on green manufacturing: it’s important, methodology and its application. Procedia Mate Sci 6:1644–1649. https://doi.org/10.1016/j.mspro.2014.07.149
Peralta-Yahya PP, Keasling JD (2010) Advanced biofuel production in microbes. Biotechnol J 5:147–162. https://doi.org/10.1002/biot.200900220
Quan L-H, Min JW, Jin Y, Wang C, Kim Y-J, Yang D-C (2012) Enzymatic biotransformation of ginsenoside Rb1 to compound K by recombinant β-glucosidase from Microbacterium esteraromaticum. J Agric Food Chem 60:3776–3781. https://doi.org/10.1021/jf300186a
Ramani G, Meera B, Vanitha C, Rao M, Gunasekaran P (2012) Production, purification, and characterization of a β-glucosidase of Penicillium funiculosum NCL1. Appl Biochem Biotechnol 167:959–972. https://doi.org/10.1007/s12010-012-9645-4
Rasmussen LE, Sørensen HR, Vind J, Viksø-Nielsen A (2006) Mode of action and properties of the β-xylosidases from Talaromyces emersonii and Trichoderma reesei. Biotechnol Bioeng 94:869–876. https://doi.org/10.1002/bit.20908
Ren S, Zhang H, Mu Y, Sun M, Liu P (2013) Pharmacological effects of astragaloside IV: a literature review. J Trad Chin Med 33:413–416. https://doi.org/10.1016/S0254-6272(13)60189-2
Rohman A, Dijkstra BW, Puspaningsih NN (2019) β-xylosidases: structural diversity, catalytic mechanism, and inhibition by monosaccharides. Int J Mol Sci 20:5524. https://doi.org/10.3390/ijms20225524
Schröder SP, De Boer C, McGregor NG, Rowland RJ, Moroz O, Blagova E, Reijngoud J, Arentshorst M, Osborn D, Morant MD (2019) Dynamic and functional profiling of xylan-degrading enzymes in Aspergillus Secretomes using activity-based probes. ACS Cent Sci 5:1067–1078. https://doi.org/10.1021/acscentsci.9b00221
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385. https://doi.org/10.1093/nar/gkg520
Shen CY, Jiang JG, Yang L, Wang DW, Zhu W (2017) Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: pharmacological mechanisms and implications for drug discovery. Br J Pharmacol 174:1395–1425. https://doi.org/10.1111/bph.13631
Singh AB (2019) Production, characteristics, and biotechnological applications of microbial xylanases. Appl Microbiol Biotechnol 103:8763–8784. https://doi.org/10.1007/s00253-019-10108-6
Sun C, Jiang M, Zhang L, Yang J, Zhang G, Du B, Ren Y, Li X, Yao J (2017) Cycloastragenol mediates activation and proliferation suppression in concanavalin A-induced mouse lymphocyte pan-activation model. Immunopharmacol Immunotoxicol 39(3):131–139. https://doi.org/10.1080/08923973.2017.1300170
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Wang L, Chen Y (2017) Efficient biotransformation of astragaloside IV to cycloastragenol by Bacillus sp. LG-502. Appl Biochem Biotechnol 183:1488–1502. https://doi.org/10.1007/s12010-017-2517-1
Wang R-F, Zheng M-M, Cao Y-D, Li H, Li C-X, Xu J-H, Wang Z-T (2015) Enzymatic transformation of vina-ginsenoside R7 to rare notoginsenoside ST-4 using a new recombinant glycoside hydrolase from Herpetosiphon aurantiacus. Appl Microbiol Biotechnol 99:3433–3442. https://doi.org/10.1007/s00253-015-6446-z
Wang Y, Chen C, Wang Q, Cao Y, Xu L, Qi R (2019) Inhibitory effects of cycloastragenol on abdominal aortic aneurysm and its related mechanisms. Br J Pharmacol 176:282–296. https://doi.org/10.1111/bph.14515
Wiederstein M, Sippl MJJN (2007) ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 35:W407–W410. https://doi.org/10.1093/nar/gkm290
Yu Y, Zhou L, Yang Y, Liu Y (2018) Cycloastragenol: an exciting novel candidate for age-associated diseases. Exp Ther Med 16:2175–2182. https://doi.org/10.3892/etm.2018.6501
Zhang YP, Sun J, Zhong JJ (2010) Biofuel production by in vitro synthetic enzymatic pathway biotransformation. Curr Opin Biotechnol 21:663–669. https://doi.org/10.1016/j.copbio.2010.05.005
Zhang S-D, Lu J-K, Yan J-Z, Zhang H (2016) Research progress of preparation technology and pharmacological effect of cycloastragenol. Chin J New Drug 16:1872–1875
Zhang C, Wang X, Zhang W, Zhao Y, Lu X (2017) Expression and characterization of a glucose-tolerant β-1, 4-glucosidase with wide substrate specificity from Cytophaga hutchinsonii. Appl Microbiol Biotechnol 101:1919–1926. https://doi.org/10.1007/s00253-016-7927-4
Zhou W, Liu X, Ye L, Feng M, Zhou P, Shi X (2014) The biotransformation of astragalosides by a novel acetyl esterase from Absidia corymbifera AS2. Process Biochem 49:1464–1471. https://doi.org/10.1016/j.procbio.2014.05.026
Zhu FM, Du B, Gao HS, Liu CJ, Li J (2010) Purification and characterization of an intracellular β-glucosidase from the protoplast fusant of Aspergillus oryzae and Aspergillus niger. Appl Biochem Microbiol 46:626–632. https://doi.org/10.1134/S0003683810060116
Acknowledgment
We thank the instrument support from Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, China.
Funding
This study was funded by the National Key Research Development Program [Grant No. 2016YFD0400601].
Author information
Authors and Affiliations
Contributions
Yuan Q and Wang W conceived and designed research. Cheng L and Zhang H conducted experiments and computational analysis. Cheng L analyzed data and wrote the manuscript. Wang W and Cui H improved the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 4833 kb)
Rights and permissions
About this article
Cite this article
Cheng, L., Zhang, H., Cui, H. et al. Efficient production of the anti-aging drug Cycloastragenol: insight from two Glycosidases by enzyme mining. Appl Microbiol Biotechnol 104, 9991–10004 (2020). https://doi.org/10.1007/s00253-020-10966-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10966-5