Abstract
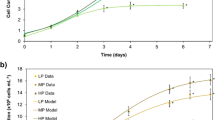
The commercial reality of microalgal biotechnology for the production of individual bioactives is constrained by the high cost of production and requires a biorefinery approach. In this investigation, we examined the influence of different nutrient deprivation (nitrogen (N), phosphorus (P), sulphur (S) and manganese (Mn)) on growth, chlorophyll a (Chl a), biohydrogen (H2) and fatty acid profiles in Parachlorella kessleri EMCCN 3073 under both aerobic and anaerobic conditions. Anaerobic conditions combined with the nutrient deprivation resulted in cell division blockage, reduction in Chl a and remarkable changes in pH, whereas a significant increase in the H2 production was observed after 24 h. The highest cumulative H2 productivity was observed in N-deficient medium (300 μL/L, day 9) followed by Mn-deficient medium (250 μL/L, day 7). The highest H2 production rate (3.37 μL/L/h) was achieved by Mn-deficient medium after 24 h. In terms of fatty acid composition, P. kessleri exhibited a differential response to different nutrient stresses. Under aerobic conditions, N-deficient media resulted in the highest lipid content (119% compared to control, day 7), whereas earlier lipid induction at (1–3 days) was observed with Mn- and S-deficient media with 18–91% and 25–34% increase, respectively, compared with the replete control. Meanwhile, higher lipid content was observed under anaerobic conditions combined with Mn-, N-, P- and S-deprived media (day 1) with 20%, 13%, 8% and 7% increases respectively compared with the control. This investigation, for the first time clearly, highlights the potential of P. kessleri as a sustainable biorefinery platform, for H2 and fatty acid bio-production under anaerobic conditions.
Key points
• Parachlorella kessleri could provide a future sustainable biorefinery platform.
• Nutrient-deprived anaerobic conditions blocked cell growth but differentially induced H 2 production.
• Nutrient status, under both aerobic/anaerobic conditions, alters lipids and fatty acids profile of P. kessleri.
• Nutrient-deprived (N- and Mn-) anaerobic conditions: future biorefinery platform.





Similar content being viewed by others
Change history
02 December 2020
A Correction to this paper has been published: https://doi.org/10.1007/s00253-020-11037-5
References
Abdel-Basset R, Friedl T, Mohr KI, Rybalka N, Martin W (2011) High growth rate, photosynthesis rate and increased hydrogen(ases) in manganese deprived cells of a newly isolated Nostoc-like cyanobacterium (SAG 2306). Int J Hydrogen Energ 36:12200–12210
Allen MM, Stanier RY (1968) Growth and division of some unicellular blue-green algae. J Gen Microbiol 51:199–202
Allen MD, Kropat J, Tottey S, Del Campo JA, Merchant SS (2006) Manganese deficiency in Chlamydomonas results in loss of photosystem II and MnSOD function, sensitivity to peroxides, and secondary phosphorus and iron deficiency. Plant Physiol 143:263–277
Batyrova KA, Tsygankov AA, Kosourov SN (2012) Sustained hydrogen photoproduction by phosphorus-deprived Chlamydomonas reinhardtii cultures. Int J Hydrogen Energ 37:8834–8839
Batyrova K, Gavrisheva A, Ivanova E, Liu J, Tsygankov A (2015) Sustainable hydrogen photoproduction by phosphorus-deprived marine green microalgae Chlorella sp. Int J Mol Sci 16:2705–2716
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226
Cakmak T, Angun P, Demiray YE, Ozkan AD, Elibol Z, Tekinay T (2012) Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol Bioeng 109:1947–1957
Cha TS, Chen JW, Goh EG, Aziz A, Loh SH (2011) Differential regulation of fatty acid biosynthesis in two Chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application. Bioresour Technol 102:10633–10640
Chen C-Y, Yeh K-L, Aisyah R, Lee D-J, Chang J-S (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Choi G-G, Kim B-H, Ahn C-Y, Oh H-M (2011) Effect of nitrogen limitation on oleic acid biosynthesis in Botryococcus braunii. J Appl Phycol 23:1031–1037
Dasgupta CN, Suseela MR, Mandotra SK, Kumar P, Pandey MK, Toppo K, Lone JA (2015) Dual uses of microalgal biomass: an integrative approach for biohydrogen and biodiesel production. Appl Energy 146:202–208
Demirbas MF (2011) Biofuels from algae for sustainable development. Appl Energy 88:3473–3480
Fernandes B, Teixeira J, Dragone G, Vicente AA, Kawano S, Bišová K, Přibyl P, Zachleder V, Vítová M (2013) Relationship between starch and lipid accumulation induced by nutrient depletion and replenishment in the microalga Parachlorella kessleri. Bioresour Technol 144:268–274
Francisco ÉC, Neves DB, Jacob-Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Ge S, Champagne P (2016) Nutrient removal, microalgal biomass growth, harvesting and lipid yield in response to centrate wastewater loadings. Water Res 88:604–612
Gfeller RP, Gibbs M (1985) Fermentative metabolism of Chlamydomonas reinhardtii: II role of Plastoquinone. Plant Physiol 77:509–511
Ghafari M, Rashidi B, Haznedaroglu BZ (2018) Effects of macro and micronutrients on neutral lipid accumulation in oleaginous microalgae. Biofuels 9:147–156
Ghirardi ML (2015) Implementation of photobiological H2 production: the O2 sensitivity of hydrogenases. Photosynth Res 125:383–393
Guan Y, Deng M, Yu X, Zhang W (2004) Two-stage photo-biological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem Eng J 19:69–73
Halim R, Gladman B, Danquah MK, Webley PA (2011) Oil extraction from microalgae for biodiesel production. Bioresour Technol 102:178–185
Hamed SM, Klöck G (2014) Improvement of medium composition and utilization of mixotrophic cultivation for green and blue green microalgae towards biodiesel production. Adv Microbiol 4:167–174
Hamed SM, Raut MP, Jaffé SRP, Wright PC (2017) Evaluation of the effect of aerobic–anaerobic conditions on photohydrogen and chlorophyll a production by environmental Egyptian cyanobacterial and green algal species. Int J Hydrogen Energ 42:6567–6577
Hase E (1962) Cell division in: Lewin RA, editor Physiol Biochem algae. Academic Press, New York, pp 617–624
He M, Li L, Liu J, Zhang L (2015) Improvement of H2 photoproduction in Chlorella pyrenoidosa in artificial and natural seawater by addition of acetic acid and control of nutrients. Algal Res 10:104–109
Hopkins TC, Graham EJS, Schwilling J, Ingram S, Gómez SM, Schuler AJ (2019) Effects of salinity and nitrogen source on growth and lipid production for a wild algal polyculture in produced water media. Algal Res 38:101406
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Iskandarov U, Khozin-Goldberg I, Cohen Z (2010) Identification and characterization of Δ;12, Δ;6, and Δ5 desaturases from the green microalga parietochloris incise. Lipids 45:519–530
Kapoore RV (2014) Mass spectrometry based hyphenated techniques for microalgal and mammalian metabolomics. Ph.D. Thesis, University of Sheffield, Sheffield, UK
Kapoore RV, Butler TO, Pandhal J, Vaidyanathan S (2018) Microwave-assisted extraction for microalgae: from biofuels to biorefinery. Biology 7:18. https://doi.org/10.3390/biology7010018
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17:36. https://doi.org/10.1186/s12934-018-0879-x
Lee DH (2011) Algal biodiesel economy and competition among bio-fuels. Bioresour Technol 102:43–49
Li Y, Han D, Sommerfeld M, Hu Q (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–129
Li X, Přibyl P, Bišová K, Kawano S, Cepák V, Zachleder V, Čížková M, Brányiková I, Vítová M (2013) The microalga Parachlorella kessleri-a novel highly efficient lipid producer. Biotechnol Bioeng 110:97–107
Li L, Zhang L, Liu J (2015) The enhancement of hydrogen photoproduction in marine Chlorella pyrenoidosa under nitrogen deprivation. Int J Hydrogen Energ 40:14784–14789
Lin Q, Lin J (2011) Effects of nitrogen source and concentration on biomass and oil production of a Scenedesmus rubescens like microalga. Bioresour Technol 102:1615–1621
Mandalam R, Palsson B (1998) Elemental balancing of biomass and medium composition enhances growth capacity in high-density Chlorella vulgaris cultures. Biotechnol Bioeng 59:605–611
Mandotra SK, Kumar P, Suseela MR, Ramteke PW (2014) Fresh water green microalga Scenedesmus abundans: a potential feedstock for high quality biodiesel production. Bioresour Technol 156:42–47
Manoyan J, Gabrielyan L, Kozel N, Trchounian A (2019) Regulation of biohydrogen production by protonophores in novel green microalgae Parachlorella kessleri. J Photochem Photobiol B Biol 199:111597
Mansour MP, Volkman JK, Blackburn SI (2003) The effect of growth phase on the lipid class, fatty acid and sterol composition in the marine dinoflagellate, Gymnodinium sp in batch culture. Phytochemistry 63:145–153
Matthew T, Zhou W, Rupprecht J, Lim L, Thomas-Hall SR, Doebbe A, Kruse O, Hankamer B, Marx UC, Smith SM, Schenk PM (2009) The metabolome of Chlamydomonas reinhardtii following induction of anaerobic H2 production by sulfur depletion. J Biol Chem 284:23415–23425
Melis A (2007) Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae). Planta 226:1075–1086
Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol 122:127–135
Mizuno Y, Sato A, Watanabe K, Hirata A, Takeshita T, Ota S, Sato N, Zachleder V, Tsuzuki M, Kawano S (2013) Sequential accumulation of starch and lipid induced by sulfur deficiency in Chlorella and Parachlorella species. Bioresour Technol 129:150–155
Mutlu YB, Işık O, Uslu L, Koç K, Durmaz Y (2011) The effects of nitrogen and phosphorus deficiencies and nitrite addition on the lipid content of Chlorella vulgaris (Chlorophyceae). Afr J Biotechnol 10:453–456
Nagy V, Vidal-Meireles A, Tengölics R, Rákhely G, Garab G, Kovács L, Tóth SZ (2016) Ascorbate accumulation during Sulphur deprivation and its effects on photosystem II activity and H2 production of the green alga Chlamydomonas reinhardtii. Plant Cell Environ 39:1460–1472
Ota S, Oshima K, Yamazaki T, Kim S, Yu Z, Yoshihara M, Takeda K, Takeshita T, Hirata A, Bišová K, Zachleder V, Hattori M, Kawano S (2016) Highly efficient lipid production in the green alga Parachlorella kessleri: draft genome and transcriptome endorsed by whole-cell 3D ultrastructure. Biotechnol Biofuels 9:13. https://doi.org/10.1186/s13068-016-0424-2
Pandhal J, Choon WL, Kapoore RV, Russo DA, Hanotu J, Wilson IAG, Desai P, Bailey M, Zimmerman WJ, Ferguson AS (2017) Harvesting environmental microalgal blooms for remediation and resource recovery: a laboratory scale investigation with economic and microbial community impact assessment. Biology (Basel) 7:4. https://doi.org/10.3390/biology7010004
Philipps G, Happe T, Hemschemeier A (2012) Nitrogen deprivation results in photosynthetic hydrogen production in Chlamydomonas reinhardtii. Planta 235:729–745
Pongpadung P, Liu J, Yokthongwattana K, Techapinyawat S, Juntawong N (2015) Screening for hydrogen-producing strains of green microalgae in phosphorus or sulphur deprived medium under nitrogen limitation. Sci Asia 41:97–107
Přibyl P, Cepák V, Zachleder V (2012) Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 94:549–561
Rashid N, Lee K, Han JI, Gross M (2013) Hydrogen production by immobilized Chlorella vulgaris: optimizing pH, carbon source and light. Bioprocess Biosyst Eng 36:867–872
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici R (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Roessler PG (1990) Enivironmental control of glycerolipid metabolism in microalgae: commercial implications and future research directions. J Phycol 26:393–399
Sadhukhan J, Ng KS, Hernandez EM (2015) Biorefineries and chemical processes : design, integration and sustainability analysis. Wiley, Hoboken
Tonon T, Harvey D, Larson TR, Graham IA (2003) Identification of a very long chain polyunsaturated fatty acid Δ4-desaturase from the microalga Pavlova lutheri. FEBS Lett 553:440–444
Veziroglu TN (2000) Quarter century of hydrogen movement 1974–2000. Int J Hydrogen Energ 25:1143–1150
Volgusheva A, Kukarskikh G, Krendeleva T, Rubin A, Mamedov F (2015) Hydrogen photoproduction in green algae Chlamydomonas reinhardtii under magnesium deprivation. RSC Adv 5:5633–5637
Wu Y-H, Yu Y, Hu H-Y (2015) Microalgal growth with intracellular phosphorus for achieving high biomass growth rate and high lipid/triacylglycerol content simultaneously. Bioresour Technol 192:374–381
Yang S, Guarnieri MT, Smolinski S, Ghirardi M, Pienkos PT (2013) De novo transcriptomic analysis of hydrogen production in the green alga Chlamydomonas moewusii through RNA-Seq. Biotechnol Biofuels 6:118. https://doi.org/10.1186/1754-6834-6-118
Yang D, Song D, Kind T, Ma Y, Hoefkens J, Fiehn O (2015) Lipidomic analysis of Chlamydomonas reinhardtii under nitrogen and sulfur deprivation. PLoS One 10(9):e0137948. https://doi.org/10.1371/journalpone0137948
Yeh KL, Chang JS (2011) Nitrogen starvation strategies and photobioreactor design for enhancing lipid content and lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: implications for biofuels. Biotechnol J 6:1358–1366
Zhang L, Happe T, Melis A (2002) Biochemical and morphological characterization of sulfur-deprived and H2-producing Chlamydomonas reinhardtii (green alga). Planta 214:552–561
Zhao W, Guo Q, Zhao J (2007) A membrane-associated Mn-superoxide dismutase protects the photosynthetic apparatus and nitrogenase from oxidative damage in the cyanobacterium Anabaena sp PCC 7120. Plant Cell Physiol 48:563–572
Funding
The authors acknowledge financial support from Science, Technology and Development fund (STDF), Egyptian State Ministry for Scientific Research with the project number 27655. Support of the EPSRC for ChELSI is also acknowledged (EP/E036252/1).
Author information
Authors and Affiliations
Contributions
SMH and RVK designed the experimental approach, analysed the data and wrote the manuscript. RVK contributed to the analysis of the lipid content in the samples. SMH and MPR contributed to the analysis of the H2 and carried out an anaerobic set of experiments. RVK and SMH generated all the figures in the manuscript. PCW and SV supervised the project and coordinated the effort. All authors wrote, read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any experiments involving human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joint first authors are Seham M. Hamed, Rahul Vijay Kapoore and Mahendra P. Raut
Electronic supplementary material
ESM 1
(PDF 71 kb)
Rights and permissions
About this article
Cite this article
Hamed, S.M., Kapoore, R.V., Raut, M.P. et al. Influence of nutrient status on the biohydrogen and lipid productivity in Parachlorella kessleri: a biorefinery approach. Appl Microbiol Biotechnol 104, 10293–10305 (2020). https://doi.org/10.1007/s00253-020-10930-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10930-3