Abstract
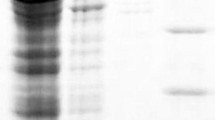
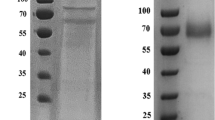
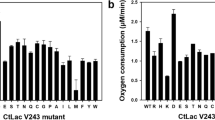
In the present study, the gene encoding a multicopper oxidase, more precisely a laccase from the thermoalkaliphilic aerobic bacterium Caldalkalibacillus thermarum strain TA2.A1 (CtLac), was cloned and expressed in Escherichia coli. CtLac is a monomeric protein with a molecular weight of 57 kDa as determined by native polyacrylamide gel electrophoresis. The optimum pH and temperature for 2,6-dimethoxyphenol (2,6-DMP) oxidation were 8.0 and 70 °C, respectively. The kinetic constants Km and kcat for 2,6-DMP were of 200 μM and 23 s−1, respectively. The enzyme was highly thermostable at 80 °C and retained more than 80% of its activity after 24 h preincubation under thermoalkaliphilic conditions. Remarkably, it showed a half-life of about 12 h at 90 °C. The enzyme activity was significantly enhanced by Cu2+ and Mn2+ and was not affected in the presence of most of the other metal ions. CtLac activity was stimulated in the presence of halides, organic solvents, and surfactants. Furthermore, the activity of CtLac on a dimeric lignin model compound, guaiacylglycerol-β-guaiacyl ether (GGGE) was investigated. Liquid chromatography-mass spectrometry analysis indicated that CtLac catalyzes dimerization of GGGE to form a C5-C5 biphenyl tetramer. The stability and activity of CtLac characterized herein under thermoalkaliphilic conditions make it a highly suitable biocatalyst for various biotechnological and industrial applications.






Similar content being viewed by others
References
Baldrian P (2006) Fungal laccases occurrence and properties. FEMS Microbiol Rev 30:215–242
Brander S, Mikkelsen JD, Kepp KP (2014) Characterization of an alkali and halide-resistant laccase expressed in E. coli: CotA from Bacillus clausii. PLoS One 2014, 9: e99402. doi:https://doi.org/10.1371/journal.pone.0099402
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Bugg TD, Ahmad M, Hardiman EM, Singh R (2011) The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol 22:394–400
Claus H (2003) Laccases and their occurrence in prokaryotes. Arch Microbiol 179:145–150
Couto R, Herrera T (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Diamantidis G, Effosse A, Potier P, Bally R (2000) Purification and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biol Biochem 32:919–927
Durao P, Chen Z, Fernandes AT, Hildebrandt P, Murgida DH, Todorovic S, Pereira MM, Melo EP, Martins LO (2008) Copper incorporation into recombinant CotA laccase from Bacillus subtilis: characterization of fully copper loaded enzymes. J Biol Inorg Chem 13:183–193
Fernandes AT, Soares CM, Pereira MM, Huber R, Grass G, Martins LO (2007) A robust metallo-oxidase from the hyperthermophilic bacterium Aquifex aeolicus. FEBS J 274:2683–2694
Freixo MR, Karmali A, Frazão C, Arteiro JM (2008) Production of laccase and xylanase from Coriolus versicolor grown on tomato pomace and their chromatographic behavior on immobilized metal chelates. Process Biochem 43:1265–1274
Gonzalo G, Colpab DI, Habibb MH, Fraaije MW (2016) Bacterial enzymes involved in lignin degradation. J Biotechnol 236:110–119
Hirai H, Nakanishi S, Nishida T (2004) Oxidative dechlorination of methoxychlor by ligninolytic enzymes from white-rot fungi. Chemosphere 55:641–645
Huang J, Wu S, Cheng H, Lei M, Liang J, Tong H (2015) Theoretical study of bond dissociation energies for lignin model compounds. J Fuel Chem Technol 43:429–436
Ihssen J, Reiss R, Luchsinger R, Thöny-Meyer L, Richter M (2015) Biochemical properties and yields of diverse bacterial laccase-like multicopper oxidases expressed in Escherichia coli. Sci Rep 5:10465–10478
Jarrell TM, Marcum CL, Sheng H, Owen BC, O’Lenick CJ, Maraun H, Bozell JJ, Kenttämaa HI (2014) Characterization of organosolv switchgrass lignin by using high performance liquid chromatography/high resolution tandem mass spectrometry using hydroxide-doped negative-ion mode electrospray ionization. Green Chem 16:2713–2727
Johannes C, Majcherczyk A (2000) Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol 66:524–528
Jones SM, Solomon EI (2015) Electron transfer and reaction mechanism of laccases. Cell Mol Life Sci 72:869–883
Kalamorz F, Keis S, McMillan DG, Olsson K, Stanton JA, Stockwell P, Black MA, Klingeman DM, Land ML, Han CS, Martin SL, Becher SA, Peddie CJ, Morgan HW, Matthies D, Preiß L, Meier T, Brown SD, Cook GM (2011) Draft genome sequence of the thermoalkaliphilic Caldalkalibacillus thermarum strain TA2.A1. J Bacteriol 193:4290–4291
Kalyani DC, Munk L, Mikkelsen JD, Meyer AS (2016) Molecular and biochemical characterization of a new thermostable bacterial laccase from Meiothermus ruber DSM 1279. RSC Adv 6:3910–3918 Kaushik G, Thakur IS (2013) Purification, characterization and usage of thermotolerant laccase from Bacillus sp. for biodegradation of synthetic dyes. Appl Biochem Microbiol 49:352–359
Kim EY, Chae HJ, Chu KH (2007) Enzymatic oxidation of aqueous pentachlorophenol. J Environ Sci (China) 19:1032–1036
Koschorreck K, Richter SM, Ene AB, Roduner E, Schmid RD, Urlacher VB (2008) Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl Microbiol Biotechnol 79:217–224
Kudanga T, Le Roes-Hill M (2014) Laccase applications in biofuels production: current status and future prospects. Appl Microbiol Biotechnol 98:6525–6542
Lu L, Wang TN, Xu TF, Wang JY, Wang CL, Zhao M (2013) Cloning and expression of thermo alkali stable laccase of Bacillus licheniformis in Pichia pastoris and its characterization. Bioresour Technol 134:81–86
Madhavi V, Lele SS (2009) Laccase: properties and applications. Bioresources 4:1694-1717 Majumdar S, Lukk T, Solbiati JO, Bauer S, Nair SK, Cronan JE, Gerlt JA (2014) Roles of small laccases from Streptomyces in lignin degradation. Biochemistry 53:4047–4058
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 277:18849–18859
Masai E, Katayama Y, Fukuda M (2007) Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotechnol Biochem 71:1–15
Mate DM, Alcalde M (2016) Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb Biotechnol 10:1457–1467
Messerschmidt A, Huber R (1990) The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin modelling and structural relationships. Eur J Biochem 187:341–352
Miyazaki K (2005) A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles 9:415–425
Morreel K, Dima O, Kim H, Lu F, Niculaes C, Vanholme R, Dauwe R, Goeminne G, Inzé D, Messens E, Ralph J, Boerjan W (2010) Mass spectrometry-based sequencing of lignin oligomers. Plant Physiol 153:1464–1478
More SS, Renuka PS, Pruthvi K, Swetha M, Malini S, Veena SM (2011) Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzyme Res 2011:7
Munk L, Sitarz AK, Kalyani DC, Mikkelsen JD, Meyer AS (2015) Can laccases catalyze bond cleavage in lignin? Biotechnol Adv 33:13–24
Murugesan K, Arulmani M, Nam IH, Kim YM, Chang YS, Kalaichelvan PT (2006) Purification and characterization of laccase produced by a white rot fungus Pleurotus sajorcaju under submerged culture condition and its potential in decolorization of azo dyes. Appl Microbiol Biotechnol 72:939–946
Nagai M, Sato T, Watanabe H, Saito K, Kawata M, Enei H (2002) Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl Microbiol Biotechnol 60:327–335
Pozdnyakova NN, Rodakiewicz-Nowak J, Turkovskaya OV (2004) Catalytic properties of yellow laccase from Pleurotus ostreatus D1. J Mol Catal B Enzym 30:19–24
Rahmanpour R, Rea D, Jamshidi S, Fülöpb V, Bugga TD (2016) Structure of Thermobifida fusca DyP-type peroxidase and activity towards kraft lignin and lignin model compounds. Arch Biochem Biophy 564:54–60
Ramalingam B, Sana B, Seayad J, Ghadessy FJ, Sullivan MB (2017) Towards understanding of laccase-catalyzed oxidative oligomerization of dimeric lignin model compounds. RSC Adv 7:11951–11958
Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM (2009) Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc Natl Acad Sci U S A 106:1760–1765
Rittstieg K, Suurnakki A, Suortti T, Kruus K, Guebitz G, Buchert J (2002) Investigations on the laccase-catalyzed polymerization of lignin model compounds using size-exclusion HPLC. Enzym Microb Technol 31:403–410
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24:219–226
Reiss R, Ihssen J, Thöny-Meyer L (2011) Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum. BMC Biotechnol 11:9–22
Ruijssenaars HJ, Hartmans S (2004) A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Appl Microbiol Biotechnol 65:177–182
Ryan S, Schnitzhofer W, Tzanov T, Cavaco-Paulo A, Gübitz GM (2003) An acid stable laccase from Sclerotium rolfsii with potential for wool dye decolorization. Enzym Microb Technol 33:766–774
Sakurai A, Masuda M, Sakakibara M (2003) Effect of surfactants on phenol removal by the method of polymerization and precipitation catalyzed by Coprinus cinereus peroxidase. J Chem Technol Biotechnol 78:952–958
Santhanam N, Vivanco JM, Decker SR, Reardon KF (2011) Expression of industrially relevant laccases: prokaryotic style. Trends Biotechnol 29:480–489
Schmidt G, Krings U, Nimtz M, Berger RG (2012) A surfactant tolerant laccase of Meripilus giganteus. World J Microbiol Biotechnol 28:1623–1632
Sondhi S, Sharma P, Saini S, Puri N, Gupta N (2014) Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS One 9:e96951. https://doi.org/10.1371/journal.pone.0096951
Telke AA, Ghodake GS, Kalyani DC, Dhanve RS, Govindwar SP (2011) Biochemical characteristics of a textile dye degrading extracellular laccase from a Bacillus sp. ADR. Bioresour Technol 102:1752–1756
Torres-Salas P, Mate DM, Ghazi I, Plou FJ, Ballesteros AO (2013) Widening the pH activity profile of a fungal laccase by directed evolution. Chem Bio Chem 14:934–937
Tuor U, Winterhalter K, Fiechter A (1995) Enzymes of white-rot fungi involved in lignin degradation and ecological determinants for wood decay. J Biotechnol 41(1):1–17
Xu F (1997) Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J Biol Chem 272:924–928
Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram UM, Solomon EI (1996) A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta 1292:303–311
Acknowledgments
This work was supported by the GIST research institute (GRI) in 2017.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Sunil Ghatge and Youri Yang contribute equally to this work and should be considered as co-first authors.
Electronic supplementary material
ESM 1
(PDF 401 kb)
Rights and permissions
About this article
Cite this article
Ghatge, S., Yang, Y., Song, WY. et al. A novel laccase from thermoalkaliphilic bacterium Caldalkalibacillus thermarum strain TA2.A1 able to catalyze dimerization of a lignin model compound. Appl Microbiol Biotechnol 102, 4075–4086 (2018). https://doi.org/10.1007/s00253-018-8898-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8898-4