Abstract
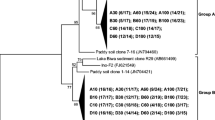
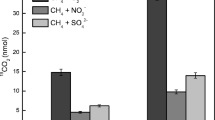
Nitrite-dependent anaerobic methane oxidation (n-damo), which is mediated by “Candidatus Methylomirabilis oxyfera-like” bacteria, is unique in linking the carbon and nitrogen cycles. However, the niche and activity of n-damo bacteria in the mangrove ecosystem have not been confirmed. Here, we report the occurrence of the n-damo process in the mangrove wetland of the Zhangjiang Estuary, China. The widespread occurrence of n-damo bacteria in mangrove wetland was confirmed using real-time quantitative polymerase chain reaction (qPCR) assay, which showed that the abundance of Methylomirabilis oxyfera-like bacterial 16S rRNA and pmoA genes ranged from 2.43 × 106 to 2.09 × 107 and 2.07 × 106 to 3.38 × 107copies per gram of dry soil in the examined sediment cores. The highest amount of targeting genes was all detected in the upper layer (0–20 cm). Phylogenetic analyses of n-damo bacterial 16S rRNA and pmoA genes illustrated the depth-specific distribution and high diversity of n-damo bacteria in the mangrove wetland. Stable isotope experiments further confirmed the occurrence of n-damo in the examined mangrove sediments, and the potential n-damo rates ranged from 25.93 to 704.08 nmol CO2 per gram of dry soil per day at different depths of the sediment cores, with the n-damo being more active in the upper layer of the mangrove sediments. These results illustrate the existence of active M. oxyfera-like bacteria and indicate that the n-damo process is a previously overlooked microbial methane sink in the mangrove wetlands.





Similar content being viewed by others
References
Alongi DM (2014) Carbon cycling and storage in mangrove forests. Annu Rev Mar Sci 6(1):195–219. https://doi.org/10.1146/annurev-marine-010213-135020
Chen J, Zhou Z, Gu JD (2015) Complex community of nitrite-dependent anaerobic methane oxidation bacteria in coastal sediments of the Mai Po wetland by PCR amplification of both 16S rRNA and pmoA genes. Appl Microbiol Biotechnol 99(3):1463–1473. https://doi.org/10.1007/s00253-014-6051-6
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Karl E, Christiane L, Likens GE (2009) Controlling eutrophication: nitrogen and phosphorus. Science 123(5917):1014–1015. https://doi.org/10.1126/science.1167755
Deutzmann JS, Schink B (2011) Anaerobic oxidation of methane in sediments of Lake Constance, an oligotrophic freshwater lake. Appl Environ Microbiol 77(13):4429–4436. https://doi.org/10.1128/AEM.00340-11
Deutzmann JS, Stief P, Brandes J, Schink B (2014) Anaerobic methane oxidation coupled to denitrification is the dominant methane sink in a deep lake. Proc Natl Acad Sci U S A 111(51):18273–18278. https://doi.org/10.1073/pnas.1411617111
Engström P, Dalsgaard T, Hulth S, Aller RC (2005) Anaerobic ammonium oxidation by nitrite (anammox): implications for N2 production in coastal marine sediments. Geochim Cosmochim Acta 69(8):2057–2065. https://doi.org/10.1016/j.gca.2004.09.032
Ettwig KF, Shima S, van de Pas-Schoonen KT, Kahnt J, Medema MH, Op den Camp HJ, Jetten MS, Strous M (2008) Denitrifying bacteria anaerobically oxidize methane in the absence of Archaea. Environ Microbiol 10(11):3164–3173. https://doi.org/10.1111/j.1462-2920.2008.01724.x
Ettwig KF, Van Alen T, Van De Pas-Schoonen KT, Jetten MS, Strous M (2009) Enrichment and molecular detection of denitrifying methanotrophic bacteria of the NC10 phylum. Appl Environ Microbiol 75(11):3656–3662. https://doi.org/10.1128/AEM.00067-09
Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MM, Schreiber F, Dutilh BE, Zedelius J, de Beer D, Gloerich J, Wessels HJ, van Alen T, Luesken F, Wu ML, van de Pas-Schoonen KT, Op den Camp HJ, Janssen-Megens EM, Francoijs KJ, Stunnenberg H, Weissenbach J, Jetten MS, Strous M (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464(7288):543–548. https://doi.org/10.1038/nature08883
Fernandes SO, Bonin PC, Michotey VD, Garcia N, Lokabharathi P (2012) Nitrogen-limited mangrove ecosystems conserve N through dissimilatory nitrate reduction to ammonium. Sci Rep-UK 2(1):419. https://doi.org/10.1038/srep00419
Finlay JC, Small GE, Sterner RW (2013) Human influences on nitrogen removal in lakes. Science 342(6155):247–250. https://doi.org/10.1126/science.1242575
Fischer H, Behrens M, Bock M, Richter U, Schmitt J, Loulergue L, Chappellaz J, Spahni R, Blunier T, Leuenberger M, Stocker TF (2008) Changing boreal methane sources and constant biomass burning during the last termination. Nature 452(7189):864–867. https://doi.org/10.1038/nature06825
Gauci V, Matthews E, Dise N, Walter B, Koch D, Granberg G, Vile M (2004) Sulfur pollution suppression of the wetland methane source in the 20th and 21st centuries. Proc Natl Acad Sci U S A 101(34):12583–12587. https://doi.org/10.1073/pnas.0404412101
Guérin F, Abril G (2007) Significance of pelagic aerobic methane oxidation in the methane and carbon budget of a tropical reservoir. J Geophys Res 112(G3). https://doi.org/10.1029/2006JG000393
Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R (2008) Error-correcting barcoded primers allow hundreds of samples to be pyrosequenced in multiplex. Nat Methods 5(3):235–237. https://doi.org/10.1038/nmeth.1184
Han P, Gu J-D (2013) A newly designed degenerate PCR primer based on pmoA gene for detection of nitrite-dependent anaerobic methane-oxidizing bacteria from different ecological niches. Appl Microbiol Biotechnol 97(23):10155–10162. https://doi.org/10.1007/s00253-013-5260-8
Haroon MF, Hu S, Shi Y, Imelfort M, Keller J, Hugenholtz P, Yuan Z, Tyson GW (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500(7464):567–570. https://doi.org/10.1038/nature12375
He Z, Geng S, Cai C, Liu S, Liu Y, Pan Y, Lou L, Zheng P, Xu X, Hu B (2015) Anaerobic oxidation of methane coupled to nitrite reduction by halophilic marine NC10 bacteria. Appl Environ Microbiol 81(16):5538–5545. https://doi.org/10.1128/AEM.00984-15
Holguin G, Guzman MA, Bashan Y (1992) Two new nitrogen-fixing bacteria from the rhizosphere of mangrove trees: their isolation, identification and in vitro interaction with rhizosphere Staphylococcus sp. FEMS Microbiol Lett 101(3):207–216. https://doi.org/10.1111/j.1574-6968.1992.tb05777.x
Hu S, Zeng RJ, Burow LC, Lant P, Keller J, Yuan Z (2009) Enrichment of denitrifying anaerobic methane oxidizing microorganisms. Environ Microbiol Rep 1(5):377–384. https://doi.org/10.1111/j.1758-2229.2009.00083.x
Hu BL, Shen LD, Lian X, Zhu Q, Liu S, Huang Q, He ZF, Geng S, Cheng DQ, Lou LP, Xu XY, Zheng P, He YF (2014) Evidence for nitrite-dependent anaerobic methane oxidation as a previously overlooked microbial methane sink in wetlands. Proc Natl Acad Sci U S A 111:4495–4500. https://doi.org/10.1073/pnas.1318393111/-/DCSupplemental
Juretschko S, Timmermann G, Schmid M, Schleifer K-H, Pommerening-Röser A, Koops H-P, Wagner M (1998) Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl Environ Microbiol 64(8):3042–3051
Kammann C, Grünhage L, Jäger HJ (2001) A new sampling technique to monitor concentrations of CH4, N2O and CO2 in air at well-defined depths in soils with varied water potential. Eur J Soil Sci 52(2):297–303. https://doi.org/10.1046/j.1365-2389.2001.00380.x
Kampman C, Hendrickx Tim LG, Luesken FA, van Alen TA, Op den Camp HJ, Jetten MS, Zeeman G, Buisman CJ, Temmink H (2012) Enrichment of denitrifying methanotrophic bacteria for application after direct low-temperature anaerobic sewage treatment. J Hazard Mater 227:164–171. https://doi.org/10.1016/j.jhazmat.2012.05.032
Kircher M, Sawyer S, Meyer M (2012) Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res 40(1):e3 1–e3 8. https://doi.org/10.1093/nar/gkr771
Kirschke S, Bousquet P, Ciais P, Saunois M, Canadell JG, Dlugokencky EJ, Bergamaschi P, Bergmann D, Blake DR, Bruhwiler L, Cameron-Smith P, Castaldi S, Chevallier F, Feng L, Fraser A, Heimann M, Hodson EL, Houweling S, Josse B, Fraser PJ, Krummel PB, Lamarque J-F, Langenfelds RL, Le Quéré C, Naik V, O'Doherty S, Palmer PI, Pison I, Plummer D, Poulter B, Prinn RG, Rigby M, Ringeval B, Santini M, Schmidt M, Shindell DT, Simpson IJ, Spahni R, Steele LP, Strode SA, Sudo K, Szopa S, van der Werf GR, Voulgarakis A, van Weele M, Weiss RF, Williams JE, Zeng G (2013) Three decades of global methane sources and sinks. Nat Geosci 6(10):813–823. https://doi.org/10.1038/NGEO1955
Knittel K, Boetius A (2009) Anaerobic oxidation of methane: progress with an unknown process. Annu Rev Microbiol 63(1):311–334. https://doi.org/10.1146/annurev.micro.61.080706.093130
Kojima H, Tsutsumi M, Ishikawa K, Iwata T, Mußmann M, Fukui M (2012) Distribution of putative denitrifying methane oxidizing bacteria in sediment of a freshwater lake, Lake Biwa. Syst Appl Microbiol 35(4):233–238. https://doi.org/10.1016/j.syapm.2012.03.005
Luesken FA, van Alen TA, van der Biezen E, Frijters C, Toonen G, Kampman C, Hendrickx TL, Zeeman G, Temmink H, Strous M, Op den Camp HJ, Jetten MS (2011a) Diversity and enrichment of nitrite-dependent anaerobic methane oxidizing bacteria from wastewater sludge. Appl Microbiol Biotechnol 92(4):845–854. https://doi.org/10.1007/s00253-011-3361-9
Luesken FA, Zhu B, van Alen TA, Butler MK, Diaz MR, Song B, Op den Camp HJ, Jetten MS, Ettwig KF (2011b) pmoA primers for detection of anaerobic methanotrophs. Appl Environ Microbiol 77(11):3877–3880. https://doi.org/10.1128/AEM.02960-10
Maie N, Pisani O, Jaffé R (2008) Mangrove tannins in aquatic ecosystems: their fate and possible influence on dissolved organic carbon and nitrogen cycling. Limnol Oceanogr 53(1):160–171. https://doi.org/10.2307/40006158
Meng H, Wang Y-F, Chan H-W, Wu R-N, Gu J-D (2016) Co-occurrence of nitrite-dependent anaerobic ammonium and methane oxidation processes in subtropical acidic forest soils. Appl Microbiol Biotechnol 100(17):7727–7739. https://doi.org/10.1007/s00253-016-7585-6
Nisbet EG, Dlugokencky EJ, Bousquet P (2014) Methane on the rise—again. Science 343(6170):493–495. https://doi.org/10.1126/science.1247828
Padilla CC, Bristow LA, Sarode N, Garcia-Robledo E, Gomez Ramirez E, Benson CR, Bourbonnais A, Altabet MA, Girguis PR, Thamdrup B, Stewart FJ (2016) NC10 bacteria in marine oxygen minimum zones. ISME J 10(8):2067–2071. https://doi.org/10.1038/ismej.2015.262
Raghoebarsing AA, Pol A, van de Pas-Schoonen KT, Smolders AJ, Ettwig KF, Rijpstra WI, Schouten S, Damste JS, Op den Camp HJ, Jetten MS, Strous M (2006) A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440(7086):918–921. https://doi.org/10.1038/nature04617
Rigby M, Prinn RG, Fraser PJ, Simmonds PG, Langenfelds RL, Huang J, Cunnold DM, Steele LP, Krummel PB, Weiss RF, O'Doherty S, Salameh PK, Wang HJ, Harth CM, Mühle J, Porter LW (2008) Renewed growth of atmospheric methane. Geophys Res Lett 35(22):L22805. https://doi.org/10.1029/2008GL036037
Segarra K, Schubotz F, Samarkin V, Yoshinaga M, Hinrichs K, Joye S (2015) High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions. Nat Commun 6:7477. https://doi.org/10.1038/ncomms8477
Shen LD, Liu S, Zhu Q, Li XY, Cai C, Cheng DQ, Lou LP, XY X, Zheng P, BL H (2014a) Distribution and diversity of nitrite-dependent anaerobic methane-oxidising bacteria in the sediments of the Qiantang River. Microb Ecol 67(2):341–349. https://doi.org/10.1007/s00248-013-0330-0
Shen LD, Liu S, Huang Q, Lian X, He Z-F, Geng S, Jin RC, He YF, Lou LP, XY X, Zheng P, BL H (2014b) Evidence for the co-occurrence of nitrite-dependent anaerobic ammonium and methane oxidation processes in a flooded paddy field. Appl Environ Microbiol 80(24):7611–7619. https://doi.org/10.1128/AEM.02379-14
Shen LD, Qun Z, Shuai L, Ping D, Jiang-Ning Z, Cheng DQ, Xu XY, Zheng P, Hu BL (2014c) Molecular evidence for nitrite-dependent anaerobic methane-oxidising bacteria in the Jiaojiang Estuary of the East Sea (China). Appl Microbiol Biotechnol 98(11):5029–5038. https://doi.org/10.1007/s00253-014-5556-3
Shen LD, Huang Q, He ZF, Lian X, Liu S, He YF, Lou LP, Xu XY, Zheng P, Hu BL (2015a) Vertical distribution of nitrite-dependent anaerobic methane-oxidising bacteria in natural freshwater wetland soils. Appl Microbiol Biot 99(1):349–357. https://doi.org/10.1007/s00253-014-6031-x
Shen LD, Liu S, He Z, Lian X, Huang Q, He Y, Lou LP, Xu XY, Zheng P, Hu BL (2015b) Depth-specific distribution and importance of nitrite-dependent anaerobic ammonium and methane-oxidising bacteria in an urban wetland. Soil Biol Biochem 83:43–51. https://doi.org/10.1016/j.soilbio.2015.01.010
Shen LD, Hu BL, Liu S, Chai XP, He ZF, Ren HX, Liu Y, Geng S, Wang W, Tang JL, Wang YM, Lou LP, Xu XY, Zheng P (2016) Anaerobic methane oxidation coupled to nitrite reduction can be a potential methane sink in coastal environments. Appl Microbiol Biotechnol 100(16):7171–7180. https://doi.org/10.1007/s00253-016-7627-0
Shindell DT, Walter BP, Faluvegi G (2004) Impacts of climate change on methane emissions from wetlands. Geophys Res Lett 31(21):L21202. https://doi.org/10.1029/2004GL021009
Shinozuka KI, Chiwa M, Nakamura K, Nagao S, Kume A (2016) Stream water nitrogen eutrophication during non-irrigated periods in a paddy-dominated agricultural basin in a snowfall area in Japan. Water Air Soil Pollut 227(7):1–11. https://doi.org/10.1007/s11270-016-2906-z
Sims G, Ellsworth T, Mulvaney R (1995) Microscale determination of inorganic nitrogen in water and soil extracts. Commun Soil Sci Plant 26(1-2):303–316. https://doi.org/10.1080/00103629509369298
Tam N, Wong A, Wong M, Wong YS (2009) Mass balance of nitrogen in constructed mangrove wetlands receiving ammonium-rich wastewater: effects of tidal regime and carbon supply. Ecol Eng 35(4):453–462. https://doi.org/10.1016/j.ecoleng.2008.05.011
Topp E, Pattey E (1997) Soils as sources and sinks for atmospheric methane. Can J Soil Sci 77(2):167–177. https://doi.org/10.4141/S96-107
Wang Y, Zhu G, Harhangi HR, Zhu B, Jetten MS, Yin C, Op den Camp HJ (2012) Co-occurrence and distribution of nitrite-dependent anaerobic ammonium and methane-oxidizing bacteria in a paddy soil. FEMS Microbiol Lett 336(2):79–88. https://doi.org/10.1111/j.1574-6968.2012.02654.x
Wang S, Wu Q, Lei T, Liang P, Huang X (2016a) Enrichment of denitrifying methanotrophic bacteria from Taihu sediments by a membrane biofilm bioreactor at ambient temperature. Environ Sci Pollut Res 23(6):5627–5634. https://doi.org/10.1007/s11356-015-5509-0
Wang Y, Huang P, Ye F, Jiang Y, Song L, Op den Camp HJ, Zhu G, Wu S (2016b) Nitrite-dependent anaerobic methane oxidizing bacteria along the water level fluctuation zone of the Three Gorges Reservoir. Appl Microbiol Biotechnol 100(4):1977–1986. https://doi.org/10.1007/s00253-015-7083-2
Ward BB, Devol AH, Rich JJ, Chang BX, Bulow SE, Naik H, Pratihary A, Jayakumar A (2009) Denitrification as the dominant nitrogen loss process in the Arabian Sea. Nature 461(7260):78–81. https://doi.org/10.1038/nature08276
Yan P, Li M, Wei G, Li H, Gao Z (2015) Molecular fingerprint and dominant environmental factors of nitrite-dependent anaerobic methane-oxidizing bacteria in sediments from the Yellow River Estuary, China. PLoS One 10(9):e0137996. https://doi.org/10.1371/journal.pone.0137996
Yang J, Jiang H, Wu G, Hou W, Sun Y, Lai Z, Dong H (2012) Co-occurrence of nitrite-dependent anaerobic methane oxidizing and anaerobic ammonia oxidizing bacteria in two Qinghai-Tibetan saline lakes. Front Earth Sci 6(4):383–391. https://doi.org/10.1007/s11707-012-0336-9
Zhou L, Wang Y, Long X-E, Guo J, Zhu G (2014) High abundance and diversity of nitrite-dependent anaerobic methane-oxidizing bacteria in a paddy field profile. FEMS MicrobiolLett 360(1):33–41. https://doi.org/10.1111/1574-6968.12567
Zhu G, Jetten MS, Kuschk P, Ettwig KF, Yin C (2010) Potential roles of anaerobic ammonium and methane oxidation in the nitrogen cycle of wetland ecosystems. Appl Microbiol Biotechnol 86(4):1043–1055. https://doi.org/10.1007/s00253-010-2451-4
Zhu B, van Dijk G, Fritz C, Smolders AJ, Pol A, Jetten MS, Ettwig KF (2012) Anaerobic oxidization of methane in a minerotrophic peatland: enrichment of nitrite-dependent methane-oxidizing bacteria. Appl Environ Microbiol 78(24):8657–8665. https://doi.org/10.1128/AEM.02102-12
Acknowledgments
We would like to thank Professor John Hodgkiss of the University of Hong Kong for correcting the English in this manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (nos. 41376117 and 41676105).
Author information
Authors and Affiliations
Contributions
YT, MPZ, and TZ designed study; MPZ, LY, LL, and WZ performed the experiments; MPZ, LY, XL, BH, MKZ, QZ, and HX analyzed data; YT and MPZ wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 423kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Luo, Y., Lin, L. et al. Molecular and stable isotopic evidence for the occurrence of nitrite-dependent anaerobic methane-oxidizing bacteria in the mangrove sediment of Zhangjiang Estuary, China. Appl Microbiol Biotechnol 102, 2441–2454 (2018). https://doi.org/10.1007/s00253-017-8718-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8718-2