Abstract
High production cost and potential pathogenicity of Pseudomonas aeruginosa, commonly used for rhamnolipid synthesis, have led to extensive research for safer producing strains and cost-effective production methods. This has resulted in numerous research publications claiming new non-pathogenic producing strains and novel production techniques many of which are unfortunately without proper characterisation of product and/or producing strain/s. Genes responsible for rhamnolipid production have only been confirmed in P. aeruginosa, Burkholderia thailandensis and Burkholderia pseudomallei. Comparing yields in different publications is also generally unreliable especially when different methodologies were used for rhamnolipid quantification. After reviewing the literature in this area, we strongly feel that numerous research outputs have insufficient evidence to support claims of rhamnolipid-producing strains and/or yields. We therefore recommend that standards should be set for reporting new rhamnolipid-producing strains and production yields. These should include (1) molecular and bioinformatic tools to fully characterise new microbial isolates and confirm the presence of the rhamnolipid rhl genes for all bacterial strains, (2) using gravimetric methods to quantify crude yields and (3) use of a calibrated method (high-performance liquid chromatography or ultra-performance liquid chromatography) for absolute quantitative yield determination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of synthetic or petrochemical-based surfactants has received considerable criticism in recent times due to their low biodegradability and environmental toxicity (Hrenovic and Ivankovic 2007; Reis et al. 2013; Scott and Jones 2000). Additionally, the use of rapidly depleting petrochemical resources in the production of synthetic surfactant is a source of some concern. These concerns have driven the search for sustainable substitutes of low toxicity obtained from renewable sources to replace synthetic surfactants. Biosurfactants, a group of naturally produced surface active compounds, are presently considered as potential substitutes for synthetic surfactants due to their biodegradability, low toxicity and ability to be produced from renewable sources of raw materials (De Almeida et al. 2016; Uzoigwe et al. 2015).
Like their synthetic counterparts, biosurfactants are surface active molecules possessing both hydrophobic and hydrophilic moieties (Banat et al. 2010; Mulligan et al. 2001; Smyth et al. 2014). They are synthesised by a wide range of microorganisms (such as bacteria, yeast and fungi) as secondary metabolites with some biosurfactants playing essential roles (such as facilitating nutrient transportation and microbe host interaction) needed for the survival of these microorganisms (Rodrigues et al. 2006; Vasileva-Tonkova et al. 2006). Different types of biosurfactants have been identified, and these have been classified into two major categories: low molecular and high molecular weight biosurfactants. The glycolipids form a class of low molecular weight biosurfactants containing some of the most studied types of biosurfactants that have shown excellent promise for commercial production and utilisation (Gautam and Tyagi 2006; Kitamoto et al. 2002; Marchant and Banat 2012a, 2012b). They are typically made up of carbohydrate molecules linked to long-chain aliphatic or hydroxyaliphatic fatty acids (Gautam and Tyagi 2006; Marchant and Banat 2012a). Within this group, rhamnolipids are some of the most promising.
As the name implies, rhamnolipids comprise rhamnose unit/units linked to 3-hydroxyl fatty acid unit/units via β-glycosidic bond. The rhamnose units are linked to each other by O-glycosidic bonds, while the 3-hydroxyl fatty acids are linked to each other by an ester bond. Rhamnolipids occur as mono-rhamnolipid and di-rhamnolipid based on the number of rhamnose units within the molecule and are further differentiated into various congeners depending on the composition of the fatty acid units (Abdel-Mawgoud et al. 2011; Abdel-Mawgoud et al. 2010; Irfan-Maqsood and Seddiq-Shams 2014).
Presently, rhamnolipids are produced mainly by Pseudomonas aeruginosa by various companies for commercial purposes (Sekhon Randhawa and Rahman 2014). They also have potential applications in a range of industries including medical, environmental, agricultural, cosmetics and pharmaceutical industries (Table 1). However, the high cost of production compared to synthetic surfactants has to a large extent hindered widespread application (De et al. 2015). As a result, present research has focused largely on identifying possible ways of reducing the cost of rhamnolipid production including the use of cheap substrates and identifying strains with high production capacity. Furthermore, the main production organism for rhamnolipid, P. aeruginosa, is classified as an opportunistic or ‘group II’ pathogen. This has raised various concerns including the risk of opportunistic infection by P. aeruginosa during large-scale industrial production (Neto et al. 2009) and the safety of the synthesised material, especially in applications involving human contact such as biomedical, cosmetics and pharmaceutical applications (Uzoigwe et al. 2015). As a result, researchers have also focused their attention on the search for suitable alternative non-pathogenic or ‘safe’ microorganisms capable of producing rhamnolipids.
The need for less expensive and safer routes for rhamnolipid production has resulted in the publication of increasing numbers of research papers on rhamnolipid production. However, many of the published works are generally unreliable, largely due to insufficient data to support the identity of the producing organism, incomplete characterisation of the product as well as poor methodology. This review is therefore aimed at evaluating the published literature on rhamnolipid production and recommending minimum guidelines for acceptance of future publications on rhamnolipid production.
Microbial rhamnolipid producers
P. aeruginosa (previously known as Pseudomonas pyocyanea) was the first reported rhamnolipid producer in the mid-twentieth century (Bergström et al. 1946). Throughout most of the twentieth century, reports on rhamnolipid production were generally from strains of Pseudomonas spp., essentially P. aeruginosa (Abdel-Mawgoud et al. 2010). Most of the research around this time was aimed at understanding the chemical structure of rhamnolipids as well as elucidating their function and mechanism of biosynthesis and regulation (Burger et al. 1963; Burger et al. 1966; Edwards and Hayashi 1965; Guerra-Santos et al. 1986; Hauser and Karnovsky 1957; Jarvis and Johnson 1949; Koch et al. 1991; Ochsner et al. 1994). However, as the need for increased production and safer microbial producers became a topic of major discussion, researchers started reporting various rhamnolipid-producing strains other than P. aeruginosa as well as strains in genera other than Pseudomonas.
A standard literature search on any of the databases will produce a list of publications with claims of biosurfactant or rhamnolipid production by various organisms, the majority of which were isolated from natural or artificial soil/water samples or industrial facilities (Kaya et al. 2014; Liu et al. 2013; Lotfabad et al. 2009; Pimienta et al. 1997; Roy et al. 2015; Saravanan and Vijayakumar 2012; Sharma et al. 2014; Wasoh 2013). However, one major problem identified in some of these cases was the lack of suitable molecular characterisation techniques for the identification of these microorganisms (Marchant et al. 2014).
Some reports based their identification mainly on physical methods of microbial characterisation such as gram staining, morphological appearances, Biolog GEN III, the analytical profile index (API) test as well as other biochemical test for microbial classification (Table 2). While these tests might be useful for quick and routine microbial characterisation, they are not sufficient for conclusive microbial identification. This is largely because the expression of certain phenotypic characteristics can vary with environmental conditions (Janda and Abbott 2002; Rossello-Mora and Amann 2001), thus leading to false positive/negative results of phenotypic traits and consequently wrong assignment of the isolated strains.
Furthermore, the use of commercially available kits to characterise microbial isolates is generally unreliable as some of these kits and their data may be outdated following identification of new species and reassignment of old ones. A clear example according to Janda and Abbott can be found in the API 20E strip, where the tests on the strip in 1975 were unchanged in 2001 (Janda and Abbott 2002).
In one case, the API 20E test and fluorescence properties were used to classify organisms identified as rhamnolipid producers as P. aeruginosa P.B:2 and Pseudomonas fluorescens P.V:10 (El-Amine et al. 2012). A recent report has however shown that isolates identified as P. fluorescens using the API 20E tests were actually Pseudomonas synxantha and Pseudomonas brassicacearum using 16S ribosomal DNA (rDNA) sequencing; these are species which are not listed on the API 20E database (Wellinghausen et al. 2005). It is certain that a comprehensive look at published reports will identify cases where isolates have been wrongly characterised; this is however beyond the scope of this review.
Sequencing 16S rDNA has greatly improved the identification and characterisation of rhamnolipid-producing isolates, but this does not come without its limitations. Previous research has suggested that 16S rDNA provides genus identification >90% in most cases and species identification at between 65 and 85% (Janda and Abbott 2007). However, in some cases where 16S identification has been used to assign species, significant phenotypic differences have been identified (Janda and Abbott 2007). This suggests that the sole use of molecular techniques to classify rhamnolipid microbial isolates is not sufficient and should be used in conjunction with suitable phenotypic and biochemical tests.
A further consideration is the quality of the 16S rDNA data used for the identification; many sequences lodged in the public databases are only partial sequences of varying lengths rather than the complete sequence generated from the cloned gene. Clearly, the shorter the partial sequence. the less reliable the identification will be.
Another problem identified in this area is that in some research where particular rhamnolipid-producing isolates have been classified as novel microbial strains, the 16S sequence of these strains is often not deposited in public databases (Zhang et al. 2000). This can lead to the assignment of multiple strain IDs to a single strain as isolates with <97% identity with strains available in public databases can be classified as new taxa (Janda and Abbott 2002).
Also, in some research where 16S rDNA sequences have been used to carry out microbial identification, results on multiple alignment and taxonomic classification are not presented either in the paper itself or in supplementary materials. In our laboratory, we have found cases of rhamnolipid-producing isolates reported as non-pathogenic strains of Pseudomonas to actually be P. aeruginosa strains. This is important because rhamnolipid production has in these cases been wrongly assigned to microbial species other than P. aeruginosa.
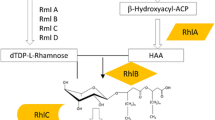
Furthermore, even when these isolates have been identified correctly, there is the problem of assigning rhamnolipid production to a particular strain without suitable molecular techniques to identify orthologs of the genes responsible for rhamnolipid production (rhlA, rhlB and rhlC orthologs) in these organisms (Marchant et al. 2014). Of all the organisms, other than P. aeruginosa, reported to produce rhamnolipid biosurfactant in the literature, only in Burkholderia spp., specifically Burkholderia thailandensis E264, Burkholderia pseudomallei and Burkholderia glumae BGR1, were the orthologs of rhlA, rhlB and rhlC identified (Costa et al. 2011; Dubeau et al. 2009) (Table 2). In these species, the three genes are grouped together in a single gene cluster which is in contrast to P. aeruginosa where rhlC is separate from rhlA and rhlB (Dubeau et al. 2009). Additionally, the gene cluster was found to be duplicated in the genomes of these organisms, except for B. glumae, with both clusters functioning in rhamnolipid production (Dubeau et al. 2009). This is interesting given that at least three species of Burkholderia have been reported to contain rhl genes with established rhamnolipid production ability. On the contrary, although the Pseudomonads have for long been considered the predominant rhamnolipid producers, only in P. aeruginosa have the genes responsible for rhamnolipid production been fully characterised.
Toribio et al. (2010) carried out a taxonomic analysis of rhl genes from four strains of P. aeruginosa (PAO1, PA14, LESB58, PA7), B. thailandensis and B. pseudomallei. They reported that the genes from these two genera belong to the same group in the phylogenetic tree, indicating that there is a low possibility of lateral gene transfer among the two genera. However, when they did a phylogenetic analysis to determine the presence of rhlA, rhlB and rhlC genes in other bacterial strains that have been reported to produce rhamnolipid, they did not find any sequence similarity in these organisms. Although, they used only those strains whose complete genome sequence is present in the public databases (Toribio et al. 2010). They further reported the presence of an rhlA ortholog in P. fluorescens SWB25, but no rhlB and rhlC orthologs were found, suggesting that rhlA might be involved in other pathways within the organism apart from rhamnolipid production (Toribio et al. 2010).
Serratia rubidaea SNAU02 was reported to be a rhamnolipid producer, and the report also claimed that the organism possesses rhamnolipid genes rhlA and rhlB (Nalini and Parthasarathi 2014). The authors reported that they sequenced the genes responsible for rhamnolipid production which they tagged rhlAB and deposited in the NCBI gene bank with accession number KF835609.1. They further reported that a blast search of the sequence showed similarity with the rhamnolipid genes of P. aeruginosa (Nalini and Parthasarathi 2014).
However, rhlAB is not a single gene but two separate genes present in a single operon in P. aeruginosa. A blast search of the gene sequence provided by the authors against the P. aeruginosa PAO1 genome indicates that this sequence is an ortholog of rhlA with 99% similarity; no similarity was found with rhlB or rhlAB operon. This shows that S. rubidaea probably only contains the rhlA ortholog with no evidence of rhlB and rhlC. Thus, there is not enough genetic evidence to support rhamnolipid production by S. rubideae.
The lack of orthologs of rhl genes in the genome sequence of these non-pseudomonas isolates available in public databases suggests that if rhamnolipid production is found in new isolates of these strains, it would have been acquired in the environment possibly through lateral transfer of genes (Toribio et al. 2010). This is most likely the case especially as these organisms are often isolated with rhamnolipid-producing organisms, particularly P. aeruginosa rhamnolipid-producing strains. It is therefore important that the rhl genes from reported rhamnolipid producers be sequenced and analysed to determine their origin. This is important for two main reasons: (i) It confirms the basis upon which rhamnolipid production is assigned and (ii) it indicates if rhamnolipid production has been acquired from the environment through gene transfer.
The second benefit is very crucial, as other researchers might want to obtain these strains from public culture collections and analyse them for rhamnolipid production. In some cases, where this has been done in our lab, we have found that these strains were not able to produce rhamnolipids. This suggested that either these strains have been wrongly identified or that isolate has acquired rhamnolipid production from the environment or the organism does not in fact produce rhamnolipids at all. Furthermore, isolated strains should be made available in public culture collections, so researchers can access these strains for further experiment. Although, it is understandable that this might not always be possible due to issues of intellectual property rights, industrial interest or patent filing.
Rhamnolipid yield measurement and congener composition
Claims of high rhamnolipid yield are gradually accumulating in the literature in response to the need to reduce rhamnolipid production costs. While this is good for the future of possible rhamnolipid exploitation in commercial products, a major concern is the assignment of yield values to microbial isolates or fermentation processes compared to what has previously being published, without taking into consideration the differences in the methods used in analysing overall rhamnolipid yield or quantifying individual rhamnolipid congener composition.
Various approaches are presently used to improve the production of rhamnolipid, including identification of high rhamnolipid-producing isolates, varying carbon and nutrient sources, varying growth and fermentation conditions, optimising recovery techniques and genetic engineering of the producing microorganisms (Camilios Neto et al. 2008; Chen et al. 2007a; Giani et al. 1996; Mukherjee et al. 2006; Rooney et al. 2009; Soares dos Santos et al. 2016). While these techniques have recorded varying rhamnolipid yields with values >100 g/L (Giani et al. 1996), a look at the methods used in quantifying the recorded yield shows that each experiment has applied different methods to quantify rhamnolipid yield and composition (Table 2). This makes it difficult to compare these methods when looking for suitable techniques to improve rhamnolipid production or to improve a particular congener composition.
A review of the different techniques presently used in quantifying general yield of rhamnolipid and congener composition is beyond the scope of this study and can be assessed in the following references (Heyd et al. 2008; Smyth et al. 2014).
Total rhamnolipid yield measurement
Rhamnolipid yields have been determined using a range of different methods; these include the use of simple gravimetric methods, colorimetric methods, infrared methods and high-performance liquid chromatography (HPLC) (Table 2). The gravimetric method is a direct and easy method to measure the weight of extracted rhamnolipid. It simply involves isolating and separating rhamnolipid and then measuring its weight.
This approach suffers from the major disadvantage that the isolated rhamnolipids are often not pure due to residual fatty acids or unspent carbon sources from the fermentation feedstock which may be extracted with the product (Abdel-Mawgoud et al. 2011; Marchant et al. 2014). This method also gives no indication of the purity of the ‘crude’ product being quantified. As stated by Marchant and Banat (2014), while solvent extraction can be used to remove most impurities, some fatty acids may not be removed and these impurities may also not be easily detected by spectrometric techniques.
Colorimetric methods have also been quite commonly used in rhamnolipid quantification. Several methods exist including orcinol assay, anthrone assay and the 6-deoxyhexose method (Smyth et al. 2014; Zhang and Miller 1992). Most colorimetric methods measure the amount of pentose sugar using a spectrophotometric assay coupled with a standard curve to quantify the amount of rhamnose in the sample which is then used to infer the quantity of the rhamnolipid by applying a multiplication factor.
These methods can also be used to measure the amount of rhamnolipid present within a fermentation broth without actually extracting the rhamnolipid, thus providing a means of monitoring yields during the fermentation processes. However, major limitations with these methods are the fact that the sugar determination is not specific for rhamnose and other sugars present will inflate the apparent value; rhamnose is present in compounds other than rhamnolipid in the cells, and finally, even with a correct determination of the quantity of rhamnose, the final estimate of rhamnolipid is made with a multiplication factor based on an estimate of the proportion of mono-rhamnolipid to di-rhamnolipid. From our experience of the orcinol assay, rhamnolipid yield values obtained from this method are always severely inflated compared to more robust quantification methods. This can result in huge discrepancies when comparing exact yields from fermentations involving different methods and microorganisms.
For example, when comparing P. aeruginosa strains known to predominantly produce Rha2-C10-C10, with B. thailandensis known to predominantly produce Rha2-C14-C14, only the rhamnose sugar will be measured. In this case, it would be inferred that the yield from both strains would be the same for each unit of rhamnose measured. However, this would be an incorrect value due to the additional eight-carbon units in each B. thailandensis rhamnolipid molecule.
Problems also occur when trying to compare yields from different experiments in which a colorimetric method was used in measuring rhamnolipid yield in one, while in others, different methods were used. For example, in their review to compare the yield of biosurfactants, including rhamnolipid, produced by different microbial strains using inexpensive carbon sources, Mukherjee et al. reported that Pseudomonas species DSM 2874 had the highest yield of 45 g/L while the other strains had yields <15 g/L (Mukherjee et al. 2006). However, close examination of the data gives a different interpretation. Trummler et al. (2003) who recorded the highest rhamnolipid yield with Pseudomonas species DSM 2874 had used a gravimetric method to estimate total rhamnolipid yield, with all the problems of uncertainty concerning the level of purity of the sample, while the other reports cited used different colorimetric methods to estimate yields. In one case, the authors of the report multiplied the rhamnose concentration by 3 in order to give an estimated total rhamnolipid yield (Nitschke et al. 2005).
In this latter case, their estimated rhamnolipid yield will be three times higher than other reported yields that have used similar colorimetric methods due to the use of the additional mathematical factor in the yield estimation. In the final analysis, a truly rigorous quantitative analysis of rhamnolipid yield can only be obtained using the type of protocol described by Rudden et al. (2015) where separation of a purified extract of rhamnolipids was carried out using ultra-performance liquid chromatography (UPLC) followed by tandem mass spectrometry to identify the individual congeners. A critical aspect of the method was the initial preparation of pure individual congener samples using flash chromatography which could then be used as quantified calibration samples. Using this method gave yield values that were considerably lower than other less accurate methods.
Product identification
The reduction of surface and interfacial tensions, emulsification properties, haemolytic activities and binding to or reactions with dyes or cationic surfactants such as cetyltrimethylammonium bromide (CTAB) are all methods employed in the screening of microbial isolates for rhamnolipid production (Heyd et al. 2008; Marchant et al. 2014; Walter et al. 2010). A major advantage of these methods is that they are quick and cheap to perform, requiring little technical expertise. Additionally, the haemolytic test and CTAB test can be carried out before setting up any fermentation run and thus can help screen out a range of isolates, saving time and additional cost.
These advantages have made these techniques very useful during the early stages of rhamnolipid research. However, a major drawback of these methods is that they are non-specific and microorganisms manufacture a wide range of metabolites which have surface active properties with very similar reactions in these tests as rhamnolipid (Heyd et al. 2008), thus giving false positive results in the search for new rhamnolipid-producing isolates.
For example, haemolytic activities can be influenced by other lytic enzymes produced by microbial isolates (Siegmund and Wagner 1991). Although CTAB was developed to curb this major disadvantage of the haemolytic test, CTAB is a harmful substance and can also limit the growth of some microorganisms (Walter et al. 2010). It is also not specific for rhamnolipid as it can be used in detecting other glycolipid and anionic surfactants (Walter et al. 2010).
Despite their drawbacks, these methods are still very useful in screening for potential biosurfactant and/or rhamnolipid producers and fulfil the criteria set out by Chen et al. (2007b) for quick screening of biosurfactant producers, which includes the ability to screen many candidates quickly, the ability to assess quantitatively the effectiveness of the surfactants and the ability to identify potential organisms. However, they are not sufficient to provide conclusive evidence that a microbial isolate does produce rhamnolipid.
To address this issue, Walter et al. (2010) recommended a combination of different methods such as the drop assay, surface and interfacial tension, haemolysis, CTAB assay, oil spreading and microplate assay for a successful screening to overcome the advantages and disadvantages of each individual method. While this recommendation helps narrow down potential rhamnolipid producers from a wide range of isolates, it should not be used as conclusive evidence, that a particular isolate does produce biosurfactants/rhamnolipid. It is therefore important that necessary fermentation, isolation and purification steps be carried out followed by other analytical techniques (such as LC-MS or LC-MS/MS) to identify the particular type of biosurfactants/rhamnolipid produced. Therefore, for a particular isolate to be reported as a rhamnolipid producer, at least one rhamnolipid congener must have been identified (in addition to the identification of the rhl genes), using any of the techniques discussed below.
Identifying rhamnolipid composition
The development of high-throughput techniques has enhanced our ability to analyse rhamnolipids. This has also led to an increase in the amount of rhamnolipid congeners known from just a few in the early 50s to over 60 (Abdel-Mawgoud et al. 2010). Techniques such as nuclear magnetic resonance (NMR), attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), liquid chromatography mass spectrometry (LC/MS) and LC/MS/MS help to precisely identify rhamnolipid and also partially quantify the various congeners present in a particular sample. A detailed description of these various techniques has previously being carried out (Leitermann et al. 2008; Smyth et al. 2014).
It is therefore important when reporting a particular isolate as a rhamnolipid producer that suitable techniques such as those mentioned above should be used to provide sufficient evidence that these isolates do produce rhamnolipid. As seen in Table 2, some reports that have claimed the production of rhamnolipid from environmental isolates have come to their conclusion using crude techniques such as haemolytic activities, CTAB assay, surface and interfacial properties and drop collapse assay, without any detailed chemical analysis of the extracts. These conclusions are not useful as results obtained from such crude analysis can only be indicative of some microbial surface active agent and not specifically rhamnolipid.
Therefore, biochemical characterisation of extracted microbial exudates presumed to be rhamnolipid biosurfactant should be carried out before it can be concluded that a particular isolate produces rhamnolipid or biosurfactant generally. Furthermore, the type of congener produced should also be reported together with their relative compositions. One advantage of knowing the congener composition is that it will help provide evidence to suggest if the particular isolate has acquired the rhamnolipid genes by lateral gene transfer. If lateral gene transfer is involved in the acquisition of rhamnolipid production ability by an isolate, the isolate will hypothetically produce rhamnolipid with congeners similar to the congeners produced by the original rhamnolipid producer from which the gene was acquired.
Recommendation and conclusion
The global market for rhamnolipid production holds great promise (Sekhon Randhawa and Rahman 2014). This dictates the need for increased industrial production and safety of the production processes used particularly for the need for safer microbial strains. However, it is essential that guidelines be implemented for publications of research results particularly those with claims of isolating rhamnolipid producers with high production capacity and non-pathogenicity. Without a satisfactory level of scientific rigour in this area, the literature will become overcrowded with reports that cannot be effectively used or relied on to advance the field. This review has considered three major areas including identification of the organism, identification of the rhl genes within the isolates and characterisation and quantification of the product. Based on the literature reviewed, we recommend that the following standards be adopted for the acceptance of publications with claims of rhamnolipid production from microbial isolate.
-
Identification of isolated strains should be carried out using routine physiological and biochemical characterisation techniques followed by 16S rDNA sequencing and phylogenetic characterisation of the isolate. The 16S rDNA sequence should wherever possible be a complete and not a partial sequence. Additional protocols for microbial identification including protocols and primers for 16S rDNA sequencing as well as protocols for phylogenetic characterisation of microbial isolates can be found in the literature (Bond et al. 2000; Stefanis et al. 2013). However, in cases where the microorganism has previously been fully characterised using at least the methods highlighted above, reference can be made to the previous study and the source of the organism stated.
-
After an isolate has been identified, bioinformatic analysis of the identified strain should be carried out using data available in public databases such as the NCBI or EMBL-EBI. In situations where the identified strain is considered to be a novel strain or where there is no complete annotation of the genome, PCR analysis should be carried out using primers of known rhl genes (such as P. aeruginosa PAO1 or B. thailandensis E264) to amplify potential rhl genes followed by sequencing of the amplified genes.
-
After suitable identification of the isolate and establishment of the presence of rhl genes have been made, fermentation experiments followed by product extraction and characterisation should be carried out. We recommend that before a particular isolate is reported to be a rhamnolipid producer, at least one rhamnolipid congener must be identified using LC/MS (using either online or offline MS) or other suitable analytical techniques such as those listed above or in the following papers (Leitermann et al. 2008; Rudden et al. 2015; Smyth et al. 2014).
-
Irrespective of the quantification method used, we recommend that extracted rhamnolipid should be measured gravimetrically to allow for general yield comparison. The gravimetric determination should also be made in conjunction with analytical methods to establish the purity of the sample being analysed. More robust standard for quantitative measurement of yield should be a chromatographic separation method followed by mass spectrometry identification of individual congeners and quantification against individual congener standards. The effect of carbon source used in rhamnolipid production should also be taken into consideration especially in reviews.
References
Abdel-Mawgoud A, Lépine F, Déziel E (2010) Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336. doi:10.1007/s00253-010-2498-2
Abdel-Mawgoud A, Hausmann R, Lépine F, Müller M, Déziel E (2011) Rhamnolipids: detection, analysis, biosynthesis, genetic regulation, and bioengineering of production. In: Soberón-Chávez G (ed) Biosurfactants, vol 20. Microbiology monographs. Springer, Berlin Heidelberg, pp 13–55. doi:10.1007/978-3-642-14490-5_2
Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87:427–444. doi:10.1007/s00253-010-2589-0
Bergström S, Theorell H, Davide H (1946) On a metabolic product of Ps. pyocyanea, pyolipic acid, active against Mycobacterium tuberculosis. Ark Chem Miner Geol 23A(13):1–12
Bond PL, Smriga SP, Banfield JF (2000) Phylogeny of microorganisms populating a thick, subaerial, predominantly Lithotrophic biofilm at an extreme acid mine drainage site. Appl Environ Microbiol 66:3842–3849. doi:10.1128/aem.66.9.3842-3849.2000
Brett PJ, DeShazer D, Woods DE (1998) Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int J Syst Bacteriol 48:317–320. doi:10.1099/00207713-48-1-317
Burger MM, Glaser L, Burton RM (1963) The enzymatic synthesis of a rhamnose-containing glycolipid by extracts of Pseudomonas aeruginosa. J of Biol Chem 238:2595–2602
Burger MM, Glaser L, Burton RM (1966) [78] Formation of rhamnolipids of Pseudomonas aeruginosa. Methods Enzymol 8:441–445. doi:10.1016/0076-6879(66)08082-0
Camilios Neto D, Meira J, de Araújo J, Mitchell D, Krieger N (2008) Optimization of the production of rhamnolipids by Pseudomonas aeruginosa UFPEDA 614 in solid-state culture. Appl Microbiol Biotechnol 81:441–448. doi:10.1007/s00253-008-1663-3
Chen SY, Lu WB, Wei YH, Chen WM, Chang JS (2007a) Improved production of biosurfactant with newly isolated Pseudomonas aeruginosa S2. Biotechnol Prog 23:661–666. doi:10.1021/bp0700152
Chen CY, Baker SC, Darton RC (2007b) The application of a high throughput analysis method for the screening of potential biosurfactants from natural sources. J Microbiol Meth 70:503–510
Christova N, Tuleva B, Lalchev Z, Jordanova A, Jordanov B (2004) Rhamnolipid biosurfactants produced by Renibacterium salmoninarum 27BN during growth on n-hexadecane. Z Naturforsch C 59:70–74
Cortés-Sánchez AJ, Hernández-Sánchez H, Jaramillo-Flores ME (2013) Biological activity of glycolipids produced by microorganisms: new trends and possible therapeutic alternatives. Microbiol Res 168:22–32. doi:10.1016/j.micres.2012.07.002
Costa SGVAO, Déziel E, Lépine F (2011) Characterization of rhamnolipid production by Burkholderia glumae. Lett Appl Microbiol 53:620–627. doi:10.1111/j.1472-765X.2011.03154.x
De Almeida DG, Soares Da Silva RCF, Luna JM, Rufino RD, Santos VA, Banat IM, Sarubbo LA (2016) Biosurfactants: promising molecules for petroleum biotechnology advances. Front Microbiol 7:1718. doi:10.3389/fmicb.2016.01718
De S, Malik S, Ghosh A, Saha R, Saha B (2015) A review on natural surfactants. RSC Adv 5:65757–65767. doi:10.1039/C5RA11101C
DeSanto K (2011) Rhamnolipid mechanism. US Patent 201,101,236,23 A1, 26 May 2011
DeSanto K (2012) Rhamnolipid-based formulations. US patent 8,183,198 B2, 22 May 2012
Dubeau D, Deziel E, Woods D, Lepine F (2009) Burkholderia thailandensis harbors two identical rhl gene clusters responsible for the biosynthesis of rhamnolipids. BMC Microbiol 9:263. doi:10.1186/1471-2180-9-263
Edwards JR, Hayashi JA (1965) Structure of a rhamnolipid from Pseudomonas aeruginosa. Arch of Biochem and Biophys 111:415–421
El-Amine B, Mebrek S, Naimi M, Tifrit A, Belaouni H (2012) Isolation and comparison of rhamnolipids production in Pseudomonas aeruginosa PB: 2 and Pseudomonas fluorescens PV: 10. Sci Rep 1:544. doi:10.4172/scientificreports.544
Fu H, Chai T, Huang G, Gao P, Liu Z (2015) Effects of rhamnolipid on the adsorption of Pb2+ onto compost humic acid. Desalin Water Treat 54:3177–3183. doi:10.1080/19443994.2014.943059
Funston SJ, Tsaousi K, Rudden M, Smyth TJ, Stevenson PS, Marchant R, Banat IM (2016) Characterising rhamnolipid production in Burkholderia thailandensis E264, a non-pathogenic producer. Appl Microbiol Biotechnol 100:7945–7956. doi:10.1007/s00253-016-7564-y
Gautam K, Tyagi V (2006) Microbial surfactants: a review. J of Oleo Sci 55:155–166. doi:10.5650/jos.55.155
Giani C, Wullbrandt D, Rothert R, Meiwes J (1996) Pseudomonas aeruginosa and its use in a process for the biotechnological preparation of L-rhamnose. US Patent, 5,501,966 A, 26 March 1996
Guerra-Santos LH, Käppeli O, Fiechter A (1986) Dependence of Pseudomonas aeruginosa continous culture biosurfactant production on nutritional and environmental factors. Appl Microbiol Biotechnol 24:443–448. doi:10.1007/bf00250320
Gunther NW, Nuñez A, Fett W, Solaiman DKY (2005) Production of rhamnolipids by Pseudomonas chlororaphis, a nonpathogenic bacterium. Appl Environ Microbiol 71:2288–2293. doi:10.1128/aem.71.5.2288-2293.2005
Hauser G, Karnovsky ML (1957) Rhamnose and rhamnolipid biosynthesis by Pseudomonas aeruginosa. J Biol Chem 224:91–105
Häußler S, Nimtz M, Domke T, Wray V, Steinmetz I (1998) Purification and characterization of a cytotoxic exolipid of Burkholderia pseudomallei. Infect Immun 66:1588–1593
Heyd M, Kohnert A, Tan TH, Nusser M, Kirschhöfer F, Brenner-Weiss G, Franzreb M, Berensmeier S (2008) Development and trends of biosurfactant analysis and purification using rhamnolipids as an example. Anal Bioanal Chem 391:1579–1590. doi:10.1007/s00216-007-1828-4
Hrenovic J, Ivankovic T (2007) Toxicity of anionic and cationic surfactant to Acinetobacter junii in pure culture. Cent Eur J Biol 2:405–414. doi:10.2478/s11535-007-0029-7
Irfan-Maqsood M, Seddiq-Shams M (2014) Rhamnolipids: well-characterized glycolipids with potential broad applicability as biosurfactants. Ind Biotechnol 10:285–291. doi:10.1089/ind.2014.0003
Janda JM, Abbott SL (2002) Bacterial identification for publication: when is enough enough? J Clin Microbiol 40:1887–1891. doi:10.1128/JCM.40.6.1887-1891.2002
Janda JM, Abbott SL (2007) 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol 45:2761–2764. doi:10.1128/jcm.01228-07
Jarvis FG, Johnson MJ (1949) A glyco-lipid produced by Pseudomonas aeruginosa. J Am Chem Soc 71:4124–4126. doi:10.1021/ja01180a073
Kaya T, Aslim B, Kariptaş E (2014) Production of biosurfactant by Pseudomonas spp. isolated from industrial waste in Turkey. Turk J of Biol 38:307–317. doi:10.3906/biy-1303-18
Kitamoto D, Isoda H, Nakahara T (2002) Functions and potential applications of glycolipid biosurfactants—from energy-saving materials to gene delivery carriers. J Biosci Bioeng 94:187–201. doi:10.1016/S1389-1723(02)80149-9
Koch AK, Käppeli O, Fiechter A, Reiser J (1991) Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J Bacteriol 173:4212–4219. doi:10.1128/jb.173.13.4212-4219.1991
Leitermann F, Syldatk C, Hausmann R (2008) Fast quantitative determination of microbial rhamnolipids from cultivation broths by ATR-FTIR spectroscopy. J Biol Eng 2:13. doi:10.1186/1754-1611-2-13
Liu J, Chen Y, Xu R, Jia Y (2013) Screening and evaluation of biosurfactant-producing strains isolated from oilfield wastewater. Indian J Microbiol 53:168–174. doi:10.1007/s12088-013-0379-y
Lotfabad TB, Shourian M, Roostaazad R, Najafabadi AR, Adelzadeh MR, Noghabi KA (2009) An efficient biosurfactant-producing bacterium Pseudomonas aeruginosa MR01, isolated from oil excavation areas in south of Iran. Colloid Surface B 69:183–193. doi:10.1016/j.colsurfb.2008.11.018
Lourith N, Kanlayavattanakul M (2009) Natural surfactants used in cosmetics: glycolipids. Int J Cosmetic Sci 31:255–261. doi:10.1111/j.1468-2494.2009.00493.x
Maier RM, Soberón-Chávez G (2000) Pseudomonas aeruginosa rhamnolipids: biosynthesis and potential applications. Appl Microbiol Biotechnol 54:625–633. doi:10.1007/s002530000443
Marchant R, Banat IM (2012a) Biosurfactants: a sustainable replacement for chemical surfactants? Biotechnol Lett 34:1597–1605. doi:10.1007/s10529-012-0956-x
Marchant R, Banat IM (2012b) Microbial biosurfactants: challenges and opportunities for future exploitation. Trends Biotechnol 30:558–565. doi:10.1016/j.tibtech.2012.07.003
Marchant R, Banat IM (2014) Protocols for measuring biosurfactant production in microbial cultures. In: Springer Protocols Handbooks. Springer, pp 1–10
Marchant R, Funston S, Uzoigwe C, Rahman P, Banat IM (2014) Production of biosurfactants from nonpathogenic bacteria. In Biosurfactants: Production and Utilization—Processes, Technologies, and Economics, CRC press, pp73–82. doi: 10.1201/b17599-7
Martinez-Toledo A, Rios-Leal E, Vazquez-Duhalt R, del Gonzalez-Chavez CM, Esparza-Garcia JF, Rodriguez-Vazquez R (2006) Role of phenanthrene in rhamnolipid production by P. putida in different media. Environ Technol 27:137–142. doi:10.1080/09593332708618628
Mukherjee S, Das P, Sen R (2006) Towards commercial production of microbial surfactants. Trends Biotechnol 24:509–515. doi:10.1016/j.tibtech.2006.09.005
Mulligan CN, Yong RN, Gibbs BF (2001) Surfactant-enhanced remediation of contaminated soil: a review. Eng Geol 60:371–380. doi:10.1016/S0013-7952(00)00117-4
Nalini S, Parthasarathi R (2014) Production and characterization of rhamnolipids produced by Serratia rubidaea SNAU02 under solid-state fermentation and its application as biocontrol agent. Bioresour Technol 173:231–238. doi:10.1016/j.biortech.2014.09.051
Neto DC, Meira JA, Tiburtius E, Zamora PP, Bugay C, Mitchell DA, Krieger N (2009) Production of rhamnolipids in solid-state cultivation: characterization, downstream processing and application in the cleaning of contaminated soils. Biotechnol J 4:748–755. doi:10.1002/biot.200800325
Nitschke M, Costa SGVAO, Haddad RG, Gonçalves LA, Eberlin MN, Contiero J (2005) Oil wastes as unconventional substrates for rhamnolipid biosurfactant production by Pseudomonas aeruginosa LBI. Biotechnol Prog 21:1562–1566. doi:10.1021/bp050198x
Nordin N, Zakaria MR, Halmi MIE, Ariff AB, Zawawi RM, Wasoh H (2013) Isolation and screening of high efficiency biosurfactant-producing bacteria Pseudomonas sp. JOBIMB 1:25–31
Ochsner UA, Koch AK, Fiechter A, Reiser J (1994) Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol 176:2044–2054
Piljac G, Piljac V (1995) Immunological activity of rhamnolipids US Patent 5,466,675 A, 14 November 1995
Piljac T, Piljac G (2007) Use of rhamnolipids as cosmetics. European Patent EP 1,056,462 B1, 25 June 2007
Piljac A, Stipcevic T, Piljac-Zegarac J, Piljac G (2008) Successful treatment of chronic decubitus ulcer with 0.1% dirhamnolipid ointment. J Cutan Med and Surg 12:142–146
Pimienta RA, Diaz MM, Caravajal SF, Grosso VJ (1997) Production of biosurfactants (Rhamnolipids) by Pseudomonas aeruginosa isolated from Colombian sludges. Ciencia TecnologíaFuturo 1:95–108
Priji P, Sajith S, Unni KN, Anderson RC, Benjamin S (2017) Pseudomonas sp. BUP6, a novel isolate from Malabari goat produces an efficient rhamnolipid type biosurfactant. J Basic Microbiol 57:21–33. doi:10.1002/jobm.201600158
Reis RS, Pacheco GJ, Pereira AG, Freire DMG (2013) Biosurfactants: production and applications. Biodegradation - Life of Science. Dr. Rolando Chamy (Ed.), InTech, doi: 10.5772/56144
Remichkova M, Galabova D, Roeva I, Karpenko E, Shulga A, Galabov AS (2008) Anti-herpesvirus activities of Pseudomonas sp. S-17 rhamnolipid and its complex with alginate. Z Naturforsch C63:75–81
Rikalović MG, Vrvić MM, Karadžić IM (2015) Rhamnolipid biosurfactant from Pseudomonas aeruginosa: from discovery to application in contemporary technology. J Serb Chem Soc 80:279–304. doi:10.2298/JSC140627096R
Rodrigues L, Banat IM, Teixeira J, Oliveira R (2006) Biosurfactants: potential applications in medicine. J Antimicrob Chemother 57:609–618. doi:10.1093/jac/dkl024
Rooney AP, Price NPJ, Ray KJ, Kuo TM (2009) Isolation and characterization of rhamnolipid-producing bacterial strains from a biodiesel facility. FEMS Microbiol Lett 295:82–87. doi:10.1111/j.1574-6968.2009.01581.x
Rossello-Mora R, Amann R (2001) The species concept for prokaryotes. FEMS Microbiol Rev 25:39–67
Roy S, Chandni S, Das I, Karthik L, Kumar G, Bhaskara Rao KV (2015) Aquatic model for engine oil degradation by rhamnolipid producing Nocardiopsis VITSISB. 3. Biotech 5:153–164. doi:10.1007/s13205-014-0199-8
Rudden M, Tsaousi K, Marchant R, Banat IM, Smyth TJ (2015) Development and validation of an ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method for the quantitative determination of rhamnolipid congeners. Appl Microbiol Biotechnol 99:9177–9187. doi:10.1007/s00253-015-6837-1
Saravanan V, Vijayakumar S (2012) Isolation and screening of biosurfactant producing microorganisms from oil contaminated soil. J Acad Indus Res 1:264–268
Scott MJ, Jones MN (2000) The biodegradation of surfactants in the environment. BBA - Biomembranes 1508:235–251. doi:10.1016/S0304-4157(00)00013-7
Sekhon Randhawa KK, Rahman PKSM (2014) Rhamnolipid biosurfactants—past, present, and future scenario of global market. Front Microbiol 5:454. doi:10.3389/fmicb.2014.00454
Sharma D, Saharan BS, Chauhan N, Bansal A, Procha S (2014) Production and structural characterization of Lactobacillus helveticus derived biosurfactant. Sci World J 2014:493548. doi:10.1155/2014/493548
Siegmund I, Wagner F (1991) New method for detecting rhamnolipids excreted by Pseudomonas species during growth on mineral agar. Biotechnol Tech 5:265–268. doi:10.1007/bf02438660
Smyth TJP, Rudden M, Tsaousi K, Marchant R, Banat IM (2014) Protocols for the detection and chemical characterisation of microbial glycolipids. In. Springer Protocols Handbooks. Humana Press, pp 1–32. doi:10.1007/8623_2014_25
Soares dos Santos A, Pereira N, Freire DMG (2016) Strategies for improved rhamnolipid production by Pseudomonas aeruginosa PA1. PeerJ 4:e2078. doi:10.7717/peerj.2078
Stanier RY, Palleroni NJ, Doudoroff M (1966) The aerobic pseudomonads a taxonomic study. J Gen Microbiol 43:159–271. doi:10.1099/00221287-43-2-159
Stefanis C, Alexopoulos A, Voidarou C, Vavias S, Bezirtzoglou E (2013) Principal methods for isolation and identification of soil microbial communities. Folia Microbiol 58:61–68. doi:10.1007/s12223-012-0179-5
Stipcevic T, Piljac T, Piljac J, Dujmic T, Piljac G (2006) Use of rhamnolipids in wound healing, treatment and prevention of gum disease and periodorntal regeneration. US Patent 7,129,218 B2, 31 October 2006
Tavares LFD, Silva PM, Junqueira M, Mariano DC, Nogueira FC, Domont GB, Freire DM, Neves BC (2013) Characterization of rhamnolipids produced by wild-type and engineered Burkholderia kururiensis. Appl Microbiol Biotechnol 97:1909–1921. doi:10.1007/s00253-012-4454-9
Toribio J, Escalante AE, Soberón-Chávez G (2010) Rhamnolipids: production in bacteria other than Pseudomonas aeruginosa. Eur J Lipid Sci Tech 112:1082–1087. doi:10.1002/ejlt.200900256
Trummler K, Effenberger F, Syldatk C (2003) An integrated microbial/enzymatic process for production of rhamnolipids and L-(+)-rhamnose from rapeseed oil with Pseudomonas sp. DSM 2874. Eur J Lipid Sci Tech 105:563–571
Tuleva Borjana K, Ivanov George R, Christova Nelly E (2002) Biosurfactant production by a new Pseudomonas putida strain. Z Naturforsch C 57:356–360. doi:10.1515/znc-2002-3-426
Uzoigwe C, Ennis C, Rahman PSM (2015) Production of biosurfactants using eco-friendly microorganisms. In: Thangavel P, Sridevi G (eds) Environmental Sustainability. Springer, India, pp 185–204. doi:10.1007/978-81-322-2056-5_11
Vasileva-Tonkova E, Galabova D, Stoimenova E, Lalchev Z (2006) Production and properties of biosurfactants from a newly isolated Pseudomonas fluorescens HW-6 growing on hexadecane. Z Naturforsch C 61:553–559
Walter V, Syldatk C, Hausmann R (2010) Screening concepts for the isolation of biosurfactant producing microorganisms. In: Sen R (ed) Biosurfactants. Springer New York, New York, pp 1–13. doi:10.1007/978-1-4419-5979-9_1
Wasoh H (2013) Isolation and screening of high efficiency biosurfactant-producing Pseudomonas sp. JOBIMB 1:25–31
Wellinghausen N, Köthe J, Wirths B, Sigge A, Poppert S (2005) Superiority of molecular techniques for identification of gram-negative, oxidase-positive rods, including morphologically nontypical Pseudomonas aeruginosa, from patients with cystic fibrosis. J Clin Microbiol 43:4070–4075. doi:10.1128/JCM.43.8.4070-4075.2005
Zhang Y, Miller RM (1992) Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant). Appl Environ Microbiol 58:3276–3282
Zhang H, Hanada S, Shigematsu T, Shibuya K, Kamagata Y, Kanagawa T, Kurane R (2000) Burkholderia kururiensis sp. nov., a trichloroethylene (TCE)-degrading bacterium isolated from an aquifer polluted with TCE. Int J Syst Evol Microbiol 50:743–749. doi:10.1099/00207713-50-2-743
Acknowledgements
VUI acknowledges the support provided by an Ulster University Vice Chancellor’s Research Scholarship.
Author information
Authors and Affiliations
Contributions
VUI wrote the initial draft. RM designed the review format and together with IMB was also involved in editing the written article together with SM and LT.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Irorere, V.U., Tripathi, L., Marchant, R. et al. Microbial rhamnolipid production: a critical re-evaluation of published data and suggested future publication criteria. Appl Microbiol Biotechnol 101, 3941–3951 (2017). https://doi.org/10.1007/s00253-017-8262-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8262-0