Abstract
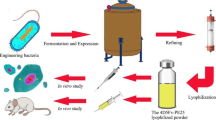
D2C7-(scdsFv)-PE38KDEL (D2C7-IT) is a novel recombinant Pseudomonas exotoxin A-based immunotoxin (IT), targeting both wild-type epidermal growth factor receptor (EGFRwt) and mutant EGFR variant III (EGFRvIII) proteins overexpressed in glioblastomas. Initial pre-clinical testing demonstrated the anti-tumor efficacy of D2C7-IT against orthotopic glioblastoma xenograft models expressing EGFRwt, EGFRvIII, or both EGFRwt and EGFRvIII. A good laboratory practice (GLP) manufacturing process was developed to produce sufficient material for a phase I/II clinical trial. D2C7-IT was expressed under the control of the T7 promoter in Escherichia coli BLR (λ DE3). D2C7-IT was produced by a 10-L batch fermentation process and was then purified from inclusion bodies using anion exchange, size exclusion, and an endotoxin removal process that achieved a yield of over 300 mg of purified protein. The final vialed batch of D2C7-IT for clinical testing was at a concentration of 0.12 ± 0.1 mg/mL, the pH was at 7.4 ± 0.4, and endotoxin levels were below the detection limit of 10 EU/mL (1.26 EU/mL). The stability of the vialed D2C7-IT has been monitored over a period of 42 months through protein concentration, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), isoelectric focusing, size exclusion chromatography, cytotoxicity, sterility, and pH measurements. The vialed D2C7-IT is currently being tested in a phase I/II clinical trial by intratumoral convection-enhanced delivery for 72 h in patients with recurrent glioblastoma (NCT02303678, D2C7 for Adult Patients with Recurrent Malignant Glioma; clinicaltrials.gov).










Similar content being viewed by others
References
Arita N, Hayakawa T, Izumoto S, Taki T, Ohnishi T, Yamamoto H, Bitoh S, Mogami H (1989) Epidermal growth factor receptor in human glioma. J Neurosurg 70(6):916–919. doi:10.3171/jns.1989.70.6.0916
Bao X, Chandramohan V, Reynolds RP, Norton JN, Wetsel WC, Rodriguiz RM, Aryal DK, McLendon RE, Levin ED, Petry NA, Zalutsky MR, Burnett BK, Kuan CT, Pastan IH, Bigner DD (2016) Preclinical toxicity evaluation of a novel immunotoxin, D2C7-(scdsFv)-PE38KDEL, administered via intracerebral convection-enhanced delivery in rats. Investig New Drugs 34(2):149–158. doi:10.1007/s10637-015-0318-3
Bartlett JM, Langdon SP, Simpson BJ, Stewart M, Katsaros D, Sismondi P, Love S, Scott WN, Williams AR, Lessells AM, Macleod KG, Smyth JF, Miller WR (1996) The prognostic value of epidermal growth factor receptor mRNA expression in primary ovarian cancer. Br J Cancer 73(3):301–306
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O'Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477. doi:10.1016/j.cell.2013.09.034
Chaffanet M, Chauvin C, Laine M, Berger F, Chedin M, Rost N, Nissou MF, Benabid AL (1992) EGF receptor amplification and expression in human brain tumours. Eur J Cancer 28(1):11–17
Chandramohan V, Bao X, Keir ST, Pegram CN, Szafranski SE, Piao H, Wikstrand CJ, McLendon RE, Kuan CT, Pastan IH, Bigner DD (2013) Construction of an immunotoxin, D2C7-(scdsFv)-PE38KDEL, targeting EGFRwt and EGFRvIII for brain tumor therapy. Clin Cancer Res 19(17):4717–4727. doi:10.1158/1078-0432.CCR-12-3891
Fox SB, Persad RA, Coleman N, Day CA, Silcocks PB, Collins CC (1994) Prognostic value of c-erbB-2 and epidermal growth factor receptor in stage A1 (T1a) prostatic adenocarcinoma. Br J Urol 74(2):214–220
Frederick L, Wang XY, Eley G, James CD (2000) Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 60(5):1383–1387
Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, Pastan I (2007) Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. Infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 13(17):5144–5149
Jiang H, Xie Y, Burnette A, Roach J, Giardina SL, Hecht TT, Creekmore SP, Mitra G, Zhu J (2013) Purification of clinical-grade disulfide stabilized antibody fragment variable—Pseudomonas exotoxin conjugate (dsFv-PE38) expressed in Escherichia coli. Appl Microbiol Biotechnol 97(2):621–632. doi:10.1007/s00253-012-4319-2
Klijn JG, Berns PM, Schmitz PI, Foekens JA (1992) The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev 13(1):3–17
Kreitman RJ, Stetler-Stevenson M, Jaffe ES, Conlon KC, Steinberg SM, Wilson W, Waldmann TA, Pastan I (2016) Complete remissions of adult T-cell leukemia with anti-CD25 recombinant immunotoxin LMB-2 and chemotherapy to block immunogenicity. Clin Cancer Res 22(2):310–318. doi:10.1158/1078-0432.CCR-15-1412
Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, Fitzgerald DJ, Wilson WH, Pastan I (2009) Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol 27(18):2983–2990. doi:10.1200/JCO.2008.20.2630
Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, Fitzgerald DJ, Lechleider R, Pastan I (2012) Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol 30(15):1822–1828. doi:10.1200/JCO.2011.38.1756
Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J (1985) Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 313(5998):144–147
Libermann TA, Razon N, Bartal AD, Yarden Y, Schlessinger J, Soreq H (1984) Expression of epidermal growth factor receptors in human brain tumors. Cancer Res 44(2):753–760
Ochiai H, Archer GE, Herndon JE 2nd, Kuan CT, Mitchell DA, Bigner DD, Pastan IH, Sampson JH (2008) EGFRvIII-targeted immunotoxin induces antitumor immunity that is inhibited in the absence of CD4+ and CD8+ T cells. Cancer Immunol Immunother 57(1):115–121. doi:10.1007/s00262-007-0363-7
Pavelic K, Banjac Z, Pavelic J, Spaventi S (1993) Evidence for a role of EGF receptor in the progression of human lung carcinoma. Anticancer Res 13(4):1133–1137
Reiter Y, Brinkmann U, Lee B, Pastan I (1996a) Engineering antibody Fv fragments for cancer detection and therapy: disulfide-stabilized Fv fragments. Nat Biotechnol 14(10):1239–1245. doi:10.1038/nbt1096-1239
Reiter Y, Pastan I (1996) Antibody engineering of recombinant Fv immunotoxins for improved targeting of cancer: disulfide-stabilized Fv immunotoxins. Clin Cancer Res 2(2):245–252
Reiter Y, Wright AF, Tonge DW, Pastan I (1996b) Recombinant single-chain and disulfide-stabilized Fv-immunotoxins that cause complete regression of a human colon cancer xenograft in nude mice. Int J Cancer 67(1):113–123. doi:10.1002/(SICI)1097-0215(19960703)67:1<113::AID-IJC19>3.0.CO;2-F
Rubin Grandis J, Melhem MF, Barnes EL, Tweardy DJ (1996) Quantitative immunohistochemical analysis of transforming growth factor-alpha and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer 78(6):1284–1292. doi:10.1002/(SICI)1097-0142(19960915)78:6<1284::AID-CNCR17>3.0.CO;2-X
Seetharam S, Chaudhary VK, FitzGerald D, Pastan I (1991) Increased cytotoxic activity of Pseudomonas exotoxin and two chimeric toxins ending in KDEL. J Biol Chem 266(26):17376–17381
Weldon JE, Pastan I (2011) A guide to taming a toxin—recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J 278(23):4683–4700. doi:10.1111/j.1742-4658.2011.08182.x
Acknowledgements
We thank Jenna Lewis for the editorial assistance of the manuscript. The study was funded by the following grant from the National Institutes of Health (NIH) of the USA: P01-CA154291-03 (to D. D. Bigner). This research was also supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author 1 declares that she has no conflict of interest. Author 2 declares that he has no conflict of interest. Author 3 declares that she has no conflict of interest. Author 4 declares that he has no conflict of interest. Author 5 declares that he has no conflict of interest. Author 6 is inventor on immunotoxin patents, all of which have been assigned to NIH. Author 7 owns stock in Istari Oncology and is a consultant to Genetron Health.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Chandramohan, V., Pegram, C.N., Piao, H. et al. Production and quality control assessment of a GLP-grade immunotoxin, D2C7-(scdsFv)-PE38KDEL, for a phase I/II clinical trial. Appl Microbiol Biotechnol 101, 2747–2766 (2017). https://doi.org/10.1007/s00253-016-8063-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-8063-x