Abstract
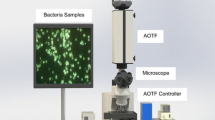
The potential for near-infrared (NIR) hyperspectral imaging and multivariate data analysis to be used as a rapid non-destructive tool for detection and differentiation of bacteria was investigated. NIR hyperspectral images were collected of Bacillus cereus, Escherichia coli, Salmonella enteritidis, Staphylococcus aureus and Staphylococcus epidermidis grown on agar for 20 h at 37 °C. Principal component analysis (PCA) was applied to mean-centred data. Standard normal variate (SNV) correction and the Savitzky-Golay technique was applied (2nd derivative, 3rd-order polynomial; 25 point smoothing) to wavelengths in the range of 1103 to 2471 nm. Chemical differences between colonies which appeared similar in colour on growth media (B. cereus, E. coli and S. enteritidis.) were evident in the PCA score plots. It was possible to distinguish B. cereus from E. coli and S. enteritidis along PC1 (59 % sum of squares (SS)) and between E. coli and S. enteritidis in the direction of PC2 (6.85 % SS). S. epidermidis was separated from B. cereus and S. aureus along PC1 (37.5 % SS) and was attributed to variation in amino acid and carbohydrate content. Two clusters were evident in the PC1 vs. PC2 PCA score plot for the images of S. aureus and S. epidermidis, thus permitting distinction between species. Differentiation between genera (similarly coloured on growth media), Gram-positive and Gram-negative bacteria and pathogenic and non-pathogenic species was possible using NIR hyperspectral imaging. Partial least squares discriminant analysis (PLS-DA) models were used to confirm the PCA data. The best predictions were made for B. cereus and Staphylococcus species, where results ranged from 82.0 to 99.96 % correctly predicted pixels.










Similar content being viewed by others
References
Alexandrakis D, Downey G, Scannell AG (2008) Detection and identification of bacteria in an isolated system with near-infrared spectroscopy and multivariate analysis. J Agric Food Chem 56(10):3431–3437
Amigo JM, Martí I, Gowen A (2013) Hyperspectral imaging and chemometrics: a perfect combination for the analysis of food structure, composition and quality. In: Marini F (ed) Chemometrics in Food Chemistry. 1st edn. Elsevier, pp 343–370
Barnes R, Dhanoa M, Lister SJ (1989) Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl Spectrosc 43(5):772–777
Bertani G (1951) Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62(3):293–300
Beveridge TJ (1999) Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol 181(16):4725–4733
Burger J (2006) Hyperspectral NIR image analysis. Swedish University of Agricultural Sciences Umeå
Chevallier S, Bertrand D, Kohler A, Courcoux P (2006) Application of PLS-DA in multivariate image analysis. J Chemometrics 20(5):221–229
Cowe IA, McNicol JW (1985) The use of principal components in the analysis of near-infrared spectra. Appl Spectrosc 39(2):257–266. doi:10.1366/0003702854248944
Davis R, Mauer L (2010) Fourier transform infrared (FT-IR) spectroscopy: a rapid tool for detection and analysis of foodborne pathogenic bacteria. In: Mendez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology. 2nd edn. Formatex Research Center, pp 1582–1594
Dissing BS, Papadopoulou OS, Tassou C, Ersbøll BK, Carstensen JM, Panagou EZ, Nychas G-J (2012) Using multispectral imaging for spoilage detection of pork meat. Food Bioprocess Technol:1–12. doi:10.1007/s11947-012-0886-6
Duan C, Chen C, Khan MN, Liu Y, Zhang R, Lin H, Cao L (2014) Non-destructive determination of the total bacteria in flounder fillet by portable near infrared spectrometer. Food Control 42:18–22. doi:10.1016/j.foodcont.2014.01.023
Dubois J, Neil Lewis E, Fry FS Jr, Calvey EM (2005) Bacterial identification by near-infrared chemical imaging of food-specific cards. Food Microbiol 22(6):577–583
Esbensen K, Geladi P (1989) Strategy of multivariate image analysis (MIA). Chemometrics Intellig Lab Syst 7(1):67–86
Feng Y-Z, ElMasry G, Sun D-W, Scannell AGM, Walsh D, Morcy N (2013) Near-infrared hyperspectral imaging and partial least squares regression for rapid and reagentless determination of Enterobacteriaceae on chicken fillets. Food Chem 138(2–3):1829–1836. doi:10.1016/j.foodchem.2012.11.040
Geladi P, Grahn H, Manley M (2010) Data analysis and chemometrics for hyperspectral imaging Raman, infrared, and near-infrared chemical imaging. Wiley, pp 93–107
Geladi P, Isaksson H, Lindqvist L, Wold S, Esbensen K (1989) Principal component analysis of multivariate images. Chemometrics Intellig Lab Syst 5(3):209–220. doi:10.1016/0169-7439(89)80049-8
Gowen AA, Feng Y, Gaston E, Valdramidis V (2015) Recent applications of hyperspectral imaging in microbiology. Talanta 137:44–53. doi:10.1016/j.talanta.2015.01.012
He H-J, Sun D-W (2015) Toward enhancement in prediction of Pseudomonas counts distribution in salmon fillets using NIR hyperspectral imaging. LWT Food Sci Technol 62(1, Part 1):11–18. doi:10.1016/j.lwt.2015.01.036
Kucheryavskiy S (2013) A new approach for discrimination of objects on hyperspectral images. Chemometrics Intellig Lab Syst 120(0):126–135. doi:10.1016/j.chemolab.2012.11.009
Lazcka O, Del Campo FJ, Munoz FX (2007) Pathogen detection: a perspective of traditional methods and biosensors. Biosensors Bioelectron 22(7):1205–1217
Liu Y, Chen Y-R, Kim MS, Chan DE, Lefcourt AM (2007) Development of simple algorithms for the detection of fecal contaminants on apples from visible/near infrared hyperspectral reflectance imaging. J Food Eng 81(2):412–418
Maity JP, Kar S, Lin C-M, Chen C-Y, Chang Y-F, Jean J-S, Kulp TR (2013) Identification and discrimination of bacteria using Fourier transform infrared spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 116:478–484. doi:10.1016/j.saa.2013.07.062
Mandal P, Biswas A, Choi K, Pal U (2011) Methods for rapid detection of foodborne pathogens: an overview. Am J Food Technol 6(2):87–102
Manley M (2014) Near-infrared spectroscopy and hyperspectral imaging: non-destructive analysis of biological materials. Chem Soc Rev 43(24):8200–8214
Mariey L, Signolle J, Amiel C, Travert J (2001) Discrimination, classification, identification of microorganisms using FTIR spectroscopy and chemometrics. Vib Spectrosc 26(2):151–159
Mingeot-Leclercq MP, Decout JL (2016) Bacterial lipid membranes as promising targets to fight antimicrobial resistance, molecular foundations and illustration through the renewal of aminoglycoside antibiotics and emergence of amphiphilic aminoglycosides. Medchemcomm 7(4):586–611. doi:10.1039/c5md00503e
Nakakimura Y, Vassileva M, Stoyanchev T, Nakai K, Osawa R, Kawano J, Tsenkova R (2012) Extracellular metabolites play a dominant role in near-infrared spectroscopic quantification of bacteria at food-safety level concentrations. Anal Methods 4(5):1389–1394
Navarre WW, Schneewind O (1999) Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev 63(1):174–229
Norris KP (1959) Infra-red spectroscopy and its application to microbiology. J Hyg 57(3):326–345
Osborne BG, Fearn T, Hindle PH (1993) Practical NIR spectroscopy with applications in food and beverage analysis, 2nd edn. Longman Scientific & Technical, Essex, England
Park B, Lawrence K, Windham W, Smith D (2004) Multispectral imaging system for fecal and ingesta detection on poultry carcasses. J Food Process Eng 27(5):311–327. doi:10.1111/j.1745-4530.2004.00464.x
Rodriguez-Saona LE, Khambaty FM, Fry FS, Calvey EM (2001) Rapid detection and identification of bacterial strains by Fourier transform near-infrared spectroscopy. J Agric Food Chem 49(2):574–579. doi:10.1021/jf000776j
Rodriguez-Saona LE, Khambaty FM, Fry FS, Dubois J, Calvey EM (2004) Detection and identification of bacteria in a juice matrix with Fourier transform-near infrared spectroscopy and multivariate analysis. J Food Prot 67(11):2555–2559
Savitzky A, Golay MJ (1964) Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 36(8):1627–1639
Schleifer KH, Kandler O (1972) Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36(2):407–477
Siripatrawan U, Makino Y, Kawagoe Y, Oshita S (2011) Rapid detection of Escherichia coli contamination in packaged fresh spinach using hyperspectral imaging. Talanta 85(1):276–281
Slavchev A, Kovacs Z, Koshiba H, Nagai A, Bázár G, Krastanov A, Kubota Y, Tsenkova R (2015) Monitoring of water spectral pattern reveals differences in probiotics growth when used for rapid bacteria selection. PLoS One 10(7):e0130698. doi:10.1371/journal.pone.0130698
Willey JM, Sherwood LM, Prescott LM (2008) Prescott, Harley, and Klein’s microbiology—7th international edition. McGraw-Hill Higher Education, New York
Williams PJ, Geladi P, Britz TJ, Manley M (2012a) Growth characteristics of three Fusarium species evaluated by near-infrared hyperspectral imaging and multivariate image analysis. Appl Microbiol Biotechnol 96(3):803–813
Williams PJ, Geladi P, Britz TJ, Manley M (2012b) Near-infrared (NIR) hyperspectral imaging and multivariate image analysis to study growth characteristics and differences between species and strains of members of the genus Fusarium. Anal Bioanal Chem 404(6–7):1759–1769
Williams PJ, Geladi P, Britz TJ, Manley M (2012c) Investigation of fungal development in maize kernels using NIR hyperspectral imaging and multivariate data analysis. J Cereal Sci 55(3):272–278. doi:10.1016/j.jcs.2011.12.003
Windham W, Yoon S-C, Ladely S, Heitschmidt J, Lawrence K, Park B, Narrang N, Cray W (2012) The effect of regions of interest and spectral pre-processing on the detection of non-0157 Shiga-toxin producing Escherichia coli serogroups on agar media by hyperspectral imaging. J Near Infrared Spectrosc 20(5):547–558
Yoon SC, Lawrence KC, Line JE, Siragusa GR, Feldner PW, Park B, Windham WR (2010) Detection of Campylobacter colonies using hyperspectral imaging. Sens & Instrumen Food Qual 4(1):35–49. doi:10.1007/s11694-010-9094-0
Yoon S-C, Windham W, Ladely S, Heitschmidt J, Lawrence K, Park B, Nareng N, Cray W (2013) Hyperspectral imaging for differentiating colonies of non-O157 Shiga-toxin producing Escherichia coli (STEC) serogroups on spread plates of pure cultures. J Near Infrared Spectrosc 21(2):81–95
Acknowledgments
This work is based on the research supported in part by the National Research Foundation of South Africa for the grant, Unique Grant No. 94031. The authors wish to thank Prof Alvaro Viljoen, Dr. Ilze Vermaak and Carmen Leonard, Tshwane University of Technology, Pretoria for use of the NIR hyperspectral imaging system and microbiology laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kammies, TL., Manley, M., Gouws, P.A. et al. Differentiation of foodborne bacteria using NIR hyperspectral imaging and multivariate data analysis. Appl Microbiol Biotechnol 100, 9305–9320 (2016). https://doi.org/10.1007/s00253-016-7801-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7801-4