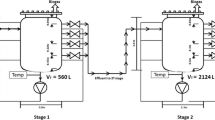
Abstract
Comparative analyses of bacterial and archaeal community structures and dynamics in three biogas digesters during start-up and subsequent operation using microwaved, ultrasonicated or untreated waste activated sludge were performed based on 454 pyrosequencing datasets of part of 16S ribosomal RNA sequences and quantitative PCR. The pre-treatment increased the solubility, and thus the availability of the substrate for microbial degradation and significantly affected the succession of the anaerobic community structure over the course of the digestion. Bacteroidetes, Proteobacteria and Firmicutes were the dominant phyla in all digesters throughout operation. Proteobacteria decreased in relative abundance from 23–26 % to 11–13 % in association with enhanced substrate availability. Negative correlations between relative abundance of Alpha-, Beta- and Gammaproteobacteria and the substrate availability and/or biogas production were disclosed in statistical analyses. Clostridiales was the dominant order in Firmicutes, and Clostridiales, Clostridia and Firmicutes relative abundance and richness were shown to positively correlate with substrate availability and biogas generation. Methanogenic communities had a fairly restricted structure, highly dominated by Methanosaeta and Methanobrevibacter phylotypes. A gradual decline in Methanobrevibacter and increased representation of Methanosaeta concilii over time were particularly apparent in the digester receiving untreated waste activated sludge, whereas more diversified archaeal communities were maintained in the pre-treatment digesters. The quantitative PCR analyses revealed a methanogenic community distribution that coincided with the 454 pyrosequencing data.





Similar content being viewed by others
References
Adewuyi YG (2001) Sonochemistry: environmental science and engineering applications. Ind Eng Chem Res 40:4681–4715. doi:10.1021/ie010096l
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Angelidaki I, Karakashev D, Batstone DJ, Plugge CM, Stams AJM (2011) Biomethane and its potential. Method Enzymol 494:327–351. doi:10.1016/B978-0-12-385112-3.00016-0
APHA (2006) Standard methods for the examination of water and wastewater, eigtheenthth edn. American Public Health Association, Washington DC
Appels L, Baeyens J, Degréve J, Dewil R (2008) Principles and potential of the anaerobic digestion of waste-activated sludge. Prog Energy Combust 34:755–781. doi:10.1016/j.pecs.2008.06.002
Appels L, Degréve J, Van der Bruggen B, Van Impe J, Dewil R (2010) Influence of low temperature thermal pre-treatment on sludge solubilisation, heavy metal release and anaerobic digestion. Bioresour Technol 101:5743–5748. doi:10.1016/j.biortech.2010.02.068
Appels L, Lauwers J, Degréve J, Helsen L, Lievens B, Willems K, Van Impe J, Dewil R (2011a) Anaerobic digestion in global bio-energy production: potential and research challenges. Renew Sust Energy Rev 15:4295–4301. doi:10.1016/j.rser.2011.07.121
Appels L, Van Assche A, Willems K, Degréve J, Van Impe J, Dewil R (2011b) Peracetic acid oxidation as an alternative pre-treatment for the anaerobic digestion of waste activated sludge. Bioresour Technol 102:4124–4130. doi:10.1016/j.biortech.2010.12.070
Azman S, Khadem AF, van Lier JB, Zeeman G, Plugge CM (2015) Presence and role of anaerobic hydrolytic microbes in conversion of lignocellulosic biomass for biogas production. Crit Rev Env Sci Tec 45:2523–2564. doi:10.1080/10643389.2015.1053727
Bates ST, Berg-Lyons D, Caporaso JG, Walters WA, Knight R, Fierer N (2011) Examining the global distribution of dominant archaeal populations in soil. ISME J 5:908–917. doi:10.1038/ismej.2010.171
Braguglia CM, Gagliano MC, Gallipoli A, Rossetti S (2012) Enhanced anaerobic digestion performances: effect of sludge ultrasound pre-treatment and role of the microbial population. Envion Engineer Manag J 11:1803–1810. doi:10.1016/j.biortech.2012.01.074
Brown SP, Veach AM, Rigdon-Huss AR, Grond K, Lickteig SK, Lothamer K, Oliver AK, Jumpponen A (2015) Scraping the bottom of the barrel: are rare high throughput sequences artifacts? Fungal Ecol 13:221–225. doi:10.1016/j.funeco.2014.08.006
Carlsson M, Lagerkvist A, Morgan-Sagastume F (2012) The effects of substrate pre-treatment on anaerobic digestion systems: a review. Waste Manag 32:1634–1650. doi:10.1016/j.wasman.2012.04.016
Carrère H, Dumas C, Battimelli A, Batstone DJ, Delgenés JP, Steyer JP, Ferrer I (2010) Pretreatment methods to improve sludge anaerobic degradability: a review. J Hazard Material 183:1–15. doi:10.1016/j.jhazmat.2010.06.129
Chen C, Wu J, Liu W (2008) Identification of important microbial populations in the mesophilic and thermophilic phenol-degrading methanogenic consortia. Water Res 42:1963–1976. doi:10.1016/j.watres.2007.11.037
Chen JL, Ortiz R, Steele TWJ (2014) Toxicants inhibiting anaerobic digestion: a review. Biotechnol Adv 32:1523–1534. doi:10.1016/j.biotechadv.2014.10.005
Cheon J, Hidaka T, Mori S, Koshikawa H, Tsuno H (2008) Applicability of random cloning method to analyze microbial community in full-scale anaerobic digesters. J Biosci Bioeng 106:134–140. doi:10.1263/jbb.106.134
Coelho NMG, Droste RL, Kennedy KJ (2011) Evaluation of continuous mesophilic, thermophilic and temperature phased anaerobic digestion of microwaved activated sludge. Water Res 45:2822–2834. doi:10.1016/j.watres.2011.02.032
Coelho NMG, Droste RL, Kennedy KJ (2014) Microwave effects on soluble substrate and thermophilic digestibility of activated sludge. Water Environ Res 86:210–222. doi:10.2175/106143013X13736496909635
De Vrieze J, Gildemyn S, Vilchez-Vargas R, Jáuregui R, Pieper DH, Verstraete W, Boon N (2015a) Inoculum selection is crucial to ensure operational stability in anaerobic digestion. Appl Microbiol Biotechnol 99:189–199. doi:10.1007/s00253-014-6046-3
De Vrieze J, Saunders AM, He Y, Fang J, Nielsen PH, Verstraete W, Boon N (2015b) Ammonia and temperature determine potential clustering in the anaerobic digestion microbiome. Water Res 75:312–323. doi:10.1016/j.watres.2015.02.025
Dearman B, Marschner P, Bentham RH (2006) Methane production and microbial community structure in single-stage batch and sequential batch systems anaerobically co-digesting food waste and biosolids. Appl Microbiol Biotechnol 69:589–596. doi:10.1007/s00253-005-0076-9
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. doi:10.1093/bioinformatics/btr381
Egli H (2008) Kjeldahl guide. Büchi Labortechnik AG, Flawil, Switzerland
Eskicioglu C, Terzian N, Kennedy KJ, Droste RL, Hamoda M (2007) Athermal microwave effects for enhancing digestibility of waste activated sludge. Water Res 41:2457–2466. doi:10.1016/j.watres.2007.03.008
EurObserv’ER (2014) Biogas barometer 2014. In: Observ’ER, Institute JS, Netherlands Ercot, Analyses IfreEE, Renac (eds). p 1-11
Gagliano MC, Braguglia CM, Gallipoli A, Gianico A, Rossetti S (2015a) Microbial diversity in innovative mesophilic/thermophilic temperature-phased anaerobic digestion of sludge. Environ Sci Pollut Res 22:7339–7348. doi:10.1007/s11356-014-3061-y
Gagliano MC, Braguglia CM, Gianico A, Mininni G, Nakamura K, Rossetti S (2015b) Thermophilic anaerobic digestion of thermal pretreated sludge: role of microbial community structure and correlation with process performances. Water Res 68:498–509. doi:10.1016/j.watres.2014.10.031
Gold ND, Martin VJJ (2007) Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis. J Bacteriol 189:6787–6795. doi:10.1128/JB.00882-07
Houtmeyers S, Degréve J, Willems K, Dewil R, Appels L (2014) Comparing the influence of low power ultrasonic and microwave pre-treatments on the solubilisation and semi-continuous anaerobic digestion of waste activated sludge. Bioresour Technol 171:44–49. doi:10.1016/j.biortech.2014.08.029
Jang HM, Cho HU, Park SK, Ha JH, Park JM (2014) Influence of thermophilic aerobic digestion as sludge pre-treatment and solids retention time of mesophilic anaerobic digestion on the methane production, sludge digestion and microbial communities in a sequential digestion process. Water Res 48:1–14. doi:10.1016/j.watres.2013.06.041
Jiang JG, Gong CX, Wang JM, Tian SC, Zhang YJ (2014) Effects of ultrasound pre-treatment on the amount of dissolved organic matter extracted from food waste. Bioresour Technol 155:266–271. doi:10.1016/j.biortech.2013.12.064
Kobayashi T, Li YY, Harada H, Yasui H, Noike T (2009) Upgrading of the anaerobic digestion of waste activated sludge by combining temperature-phased anaerobic digestion and intermediate ozonation. Water Sci Technol 59:185–193. doi:10.2166/wst.2009.510
Laurent J, Casellas M, Dagot C (2010) Heavy metals biosorption on disintegrated activated sludge: description of a new equilibrium model. Chem Eng J 164:63–69. doi:10.1016/j.cej.2010.08.023
Lee S, Kang H, Lee YH, Lee TJ, Han K, Choi Y, Park H (2012) Monitoring bacterial community structure and variability in time scale in full-scale anaerobic digesters. J Environ Monit 14:1893–1905. doi:10.1039/c2em10958a
Levén L, Schnürer A (2010) Molecular characterisation of two anaerobic phenol-degrading enrichment cultures. Int Biodeterior Biodegrad 64:427–433. doi:10.1016/j.ibiod.2010.04.009
Liu Y, Balkwill DL, Aldrich HC, Drake GR, Boone DR (1999) Characterization of the anaerobic propionate-degrading syntrophs Smithella propionica gen. nov., sp. nov. and Syntrophobacter wolinii. Inter J Syst Bacteriol 49:545–556
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Molecul Biolog Rev 66:506–577. doi:10.1128/MMBR.66.3.506-577.2002
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17:10-12 doi: 10.14806/ej.17.1.200
McInerney MJ, Bryant MP, Hespell RB, Costerton JW (1981) Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic, syntrophic, fatty acid-oxidizing bacterium. Appl Environ Microbiol 41:1029–1039
McInerney MJ, Struchtemeyer CG, Sieber J, Mouttaki H, Stams AJM, Schink B, Rohlin L, Gunsalus RP (2008) Physiology, ecology, phylogeny and genomics of microorganisms capable of syntrophic metabolism. Ann N Y Acad Sci 1125:58–72. doi:10.1196/annals.1419.005
Monlau F, Sambusiti C, Barakat A, Quéméneur M, Trably E, Steyer JP, Carrère H (2014) Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol Adv 32:934–951. doi:10.1016/j.biotechadv.2014.04.007
More TT, Yadav JSS, Yan S, Tyagi RD, Surampalli RY (2014) Extracellular polymeric substances of bacteria and their potential environmental applications. J Environ Manag 144:1–25. doi:10.1016/j.jenvman.2014.05.010
Mudhoo A, Sharma SK (2011) Microwave irradiation technology in waste sludge and wastewater treatment research. Crit Rev Environ Sci Technol 41:999–1066. doi:10.1080/10643380903392767
Murray WD (1986) Cellulose hydrolysis by Bacteroides cellulosolvens. Biomass 10:47–57. doi:10.1016/0144-4565(86)90035-1
Nishiyama T, Ueki A, Kaku N, Watanabe K, Ueki K (2009) Bacteroides graminisolvens sp. nov., a xylanolytic anaerobe isolated from a methanogenic reactor treating cattle waste. Int J Syst Evol Microbiol 59:1901–1907. doi:10.1099/ijs.0.008268-0
Oksanen J, Blanchet G, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) Community ecology package. vol R package version 2.3-0,
Park SK, Jang HM, Ha JH, Park JM (2014) Sequential sludge digestion after diverse pre-treatment conditions: sludge removal, methane production and microbial community changes. Bioresour Technol 162:331–340. doi:10.1016/j.biortech.2014.03.152
Pervin HM, Dennis PG, Lim HJ, Tyson GW, Batstone DJ, Bond PL (2013) Drivers of microbial community composition in mesophilic and thermophilic temperature-phased anaerobic digestion pre-treatment reactors. Water Res 47:7098–7108. doi:10.1016/j.watres.2013.07.053
Qiu YL, Hanada S, Ohashi A, Harada H, Kamagata Y, Sekiguchi Y (2008) Syntrophorhabdus aromaticivorans gen. nov., sp. nov., the first cultured anaerobe capable of degrading phenol to acetate in obligate syntrophic associations with a hydrogenotrophic methanogen. Appl Environ Microbiol 74:2051–2058. doi:10.1128/AEM.02378-07
Ramsay IR, Pullammanappallil PC (2001) Protein degradation during anaerobic wastewater treatment: derivation of stoichiometry. Biodegradation 12:247–257
Regueiro L, Veiga P, Figueroa M, Alonso-Gutierrez J, Stams AJM, Lema JM, Carballa M (2012) Relationship between microbial activity and microbial community structure in six full-scale anaerobic digesters. Microbiol Res 167:581–589. doi:10.1016/j.micres.2012.06.002
Roy F, Samain E, Dubourguier HC, Albagnac G (1986) Syntrophomonas sapovorans sp. nov., a new obligately proton reducing anaerobe oxidizing saturated and unsaturated long chain fatty acids. Arch Microbiol 145:142–147
Schattauer A, Abdoun E, Weiland P, Plöchl M, Heiermann M (2011) Abundance of trace elements in demonstration biogas plants. Biosyst Engineer 108:57–65. doi:10.1016/j.biosystemseng.2010.10.010
Schloss PD, Westcott SL, Ryanbin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2012) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi:10.1128/AEM.01541-09
Schlüter A, Bekel T, Diaz NN, Dondrup M, Eichenlaub R, Gartemann K, Krahn I, Krause L, Krömeke H, Kruse O, Mussgnug JH, Neuweger H, Niehaus K, Pühler A, Runte KJ, Szczepanowski R, Tauch A, Tilker A, Viehöver P, Goesmann A (2008) The metagenome of a biogas-producing microbial community of a production-scale biogas plant fermenter analysed by the 454-pyrosequencing technology. J Biotechnol 136:77–90
Shiratori H, Sasaya K, Ohiwa H, Ikeno H, Ayame S, Kataoka N, Miya A, Beppu T, Ueda K (2009) Clostridium clariflavum sp. nov. and Clostridium caenicola sp nov., moderately thermophilic, cellulose-/cellobiose-digesting bacteria isolated from methanogenic sludge. Int J Syst Evol Microbiol 59:1764–1770. doi:10.1099/ijs.0.003483-0
Smith KS, Ingram-Smith C (2007) Methanosaeta, the forgotten methanogen? Trends Microbiol 15:150–155. doi:10.1016/j.tim.2007.02.002
Stasinakis AS (2012) Review on the fate of emerging contaminants during sludge anaerobic digestion. Bioresour Technol 121:432–440. doi:10.1016/j.biortech.2012.06.074
Sun R, Xing D, Jia J, Zhou A, Zhang L, Ren N (2014) Methane production and microbial community structure for alkaline pretreated waste activated sludge. Bioresour Technol 169:496–501. doi:10.1016/j.biortech.2014.07.032
Sundberg C, Al-Soud WA, Larsson M, Alm EJ, Yekta SS, Svensson BH, Sorensen SJ, Karlsson A (2013) 454 pyrosequencing analyses of bacterial and archaeal richness in 21 full-scale biogas digesters. FEMS Microbiol Ecol 85:612–626. doi:10.1111/1574-6941.12148
Tiehm A, Nickel K, Zellhorn M, Neis U (2001) Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Res 35:2003–2009. doi:10.1016/S0043-1354(00)00468-1
Tyagi VK, Lo S, Appels L, Dewil R (2014) Ultrasonic treatment of waste sludge: a review on mechanisms and applications. Crit Rev Envrion Sci Technol 44:1220–1288. doi:10.1080/10643389.2013.763587
Vanwonterghem I, Jensen P, Dennis PG, Hugenholtz P, Rabaey K, Tyson GW (2014) Deterministic processes guide long-term synchronised population dynamics in replicate anaerobic digesters. ISME J 8:2015–2028. doi:10.1038/ismej.2014.50
Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman W (2009) The Firmicutes Bergey’s manual of systematic bacteriology. vol 3. Springer, New York, pp 1-7
Waud M, Busschaert P, Ruyters S, Jacquemyn H, Lievens B (2014) Impact of primer choice on characterization of orchid mycorrhizal communities using 454 pyrosequencing. Mol Ecol Resour 14:679–699. doi:10.1111/1755-0998.12229
Wei N (2012) Effect of ultrasonic pretreatment for the anaerobic digestion of sewage sludge. Adv Mat Res 531:528–531. doi:10.4028/http://www.scientific.net/AMR.531.528
Weiss A, Jerome V, Freitag R, Mayer HK (2008) Diversity of the resident microbiota in a thermophilic municipal biogas plant. Appl Microbiol Biotechnol 81:163–173
Westerholm M, Dolfing J, Sherry A, Gray ND, Head IM, Schnürer A (2011) Quantification of syntrophic acetate-oxidizing microbial communities in biogas processes. Environ Microbiol Reports 3:500–505. doi:10.1111/j.1758-2229.2011.00249.x
Westerholm M, Levén L, Schnürer A (2012) Bioaugmentation of syntrophic acetate-oxidizing culture in biogas reactors exposed to increasing levels of ammonia. Appl Environ Microbiol 78(21):7619–7625. doi:10.1128/AEM.01637-12
Worm P, Koehorst JJ, Visser M, Sedano-Núnez VT, Schaap PJ, Plugge CM, Sousa DZ, Stams AJM (2014) A genomic view on syntrophic versus non-syntrophic lifestyle in anaerobic acid degrading communities. Biochimica et Biophysica Acta vol 1837:2004–2016
Yang Y, Yu K, Xia Y, Lau FTK, Tang DTW, Fung WC, Fang HHP, Zhang T (2014) Metagenomic analysis of sludge from full-scale anaerobic digesters operated in municipal wastewater treatment plants. Appl Microbiol Biotechnol 98:5709–5718. doi:10.1007/s00253-014-5648-0
Yenigün O, Demirel B (2013) Ammonia inhibition in anaerobic digestion: a review. Process Biochem 48:901–911. doi:10.1016/j.procbio.2013.04.012
Yue Z, Chen R, Yang F, MacLellan J, Marsh T, Liu Y, Liao W (2013) Effects of dairy manure and corn stover co-digestion on anaerobic microbes and corresponding digestion performance. Bioresour Technol 128:65–71. doi:10.1016/j.biortech.2012.10.115
Acknowledgments
The authors would like to thank the Research Council of KU Leuven (projects OT/13/063 and F+/14/037) and the Industrial Research Council of KU Leuven (KP/10/006) for the financial support. We especially thank Sofie Houtmeyers for providing the digester samples, Stefan Ruyters for helping with the preparation of the 454 pyrosequencing run and Ken Meerbergen for assisting in some of the qPCR analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Esm 1
(PDF 835 kb)
Rights and permissions
About this article
Cite this article
Westerholm, M., Crauwels, S., Van Geel, M. et al. Microwave and ultrasound pre-treatments influence microbial community structure and digester performance in anaerobic digestion of waste activated sludge. Appl Microbiol Biotechnol 100, 5339–5352 (2016). https://doi.org/10.1007/s00253-016-7321-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7321-2