Abstract
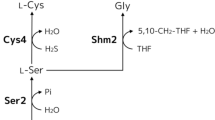
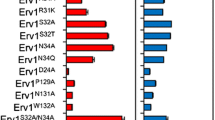
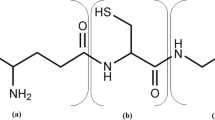
Glutathione is a valuable tripeptide widely used in the pharmaceutical, food, and cosmetic industries. In industrial fermentation, glutathione is currently produced primarily using the yeast Saccharomyces cerevisiae. Intracellular glutathione exists in two forms; the majority is present as reduced glutathione (GSH) and a small amount is present as oxidized glutathione (GSSG). However, GSSG is more stable than GSH and is a more attractive form for the storage of glutathione extracted from yeast cells after fermentation. In this study, intracellular GSSG content was improved by engineering thiol oxidization metabolism in yeast. An engineered strain producing high amounts of glutathione from over-expression of glutathione synthases and lacking glutathione reductase was used as a platform strain. Additional over-expression of thiol oxidase (1.8.3.2) genes ERV1 or ERO1 increased the GSSG content by 2.9-fold and 2.0-fold, respectively, compared with the platform strain, without decreasing cell growth. However, over-expression of thiol oxidase gene ERV2 showed almost no effect on the GSSG content. Interestingly, ERO1 over-expression did not decrease the GSH content, raising the total glutathione content of the cell, but ERV1 over-expression decreased the GSH content, balancing the increase in the GSSG content. Furthermore, the increase in the GSSG content due to ERO1 over-expression was enhanced by additional over-expression of the gene encoding Pdi1, whose reduced form activates Ero1 in the endoplasmic reticulum. These results indicate that engineering the thiol redox metabolism of S. cerevisiae improves GSSG and is critical to increasing the total productivity and stability of glutathione.




Similar content being viewed by others
References
Chen DC, Yang BC, Kuo TT (1992) One-step transformation of yeast in stationary phase. Curr Genet 21:83–84
Dröge W, Breitkreutz R (2000) Glutathione and immune function. Proc Nutr Soc 59:595–600
Finley JW, Wheeler EL, Witt SC (1981) Oxidation of glutathione by hydrogen peroxide and other oxidizing agents. J Agric Food Chem 29:404–407
Flohé L (1985) The glutathione peroxidase reaction: molecular basis of the antioxidant function of selenium in mammals. Curr Top Cell Regul 27:473–478
Frand AR, Kaiser CA (1998) The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell 1:161–170
Frand AR, Kaiser CA (1999) Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol Cell 4:469–477
Gerber J, Mühlenhoff U, Hofhaus G, Lill R, Lisowsky T (2001) Yeast ERV2p is the first microsomal FAD-linked sulfhydryl oxidase of the Erv1p/Alrp protein family. J Biol Chem 276:23486–23491
Grant CM, Dawes IW (1996) Synthesis and role of glutathione in protection against oxidative stress in yeast. Redox Rep 2:223–229
Hara KY, Kiriyama K, Inagaki A, Nakayama H, Kondo A (2012) Improvement of glutathione production by metabolic engineering the sulfate assimilation pathway of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 94:1313–1319
Hara KY, Araki M, Okai N, Wakai S, Hasunuma T, Kondo A (2014) Development of bio-based fine chemical production through synthetic bioengineering. Microb Cell Factories 13:173
Ishii J, Izawa K, Matsumura S, Wakamura K, Tanino T, Tanaka T, Ogino C, Fukuda H, Kondo A (2009) A simple and immediate method for simultaneously evaluating expression level and plasmid maintenance in yeast. J Biochem 145:701–708
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Kim S, Sideris DP, Sevier CS, Kaiser CA (2012) Balanced Ero1 activation and inactivation establishes ER redox homeostasis. J Cell Biol 196:713–725
Kiriyama K, Hara KY, Kondo A (2013) Oxidized glutathione fermentation using Saccharomyces cerevisiae engineered for glutathione metabolism. Appl Microbiol Biotechnol 97:7399–7404
Li Y, Wei G, Chen J (2004) Glutathione: a review on biotechnological production. Appl Microbiol Biotechnol 66:233–242
Lin SK, Tsai SM, Huang JC, Lee SC, Wu SH, Wu SH, Ma H, Lin JT, Tsai LY (2006) Effects of storage time and temperature on the stability of glutathione in deproteinized blood sample. J Food Drug Anal 14:141–146
Meister A, Andersen ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Mesecke N, Terziyska N, Kozany C, Baumann F, Neupert W, Hell K, Herrmann JM (2005) A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 121:1059–1069
Morgan B, Ezeriņa D, Amoako TN, Riemer J, Seedorf M, Dick TP (2013) Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nat Chem Biol 9:119–125
Penninckx MJ (2002) An overview on glutathione in Saccharomyces versus non-conventional yeasts. FEMS Yeast Res 2:295–305
Pócsi I, Prade RA, Penninckx MJ (2004) Glutathione, altruistic metabolite in fungi. Adv Microb Physiol 49:1–76
Pollard MG, Travers KJ, Weissman JS (1998) Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol Cell 1:171–182
Ray S, Watkins DN, Misso NL, Thompson PJ (2002) Oxidant stress induces gamma-glutamylcysteine synthetase and glutathione synthesis in human bronchial epithelial NCI-H292 cells. Clin Exp Allergy 32:571–577
Rolseth V, Djurhuus R, Svardal AM (2002) Additive toxicity of limonene and 50% oxygen and the role of glutathione in detoxification in human lung cells. Toxicology 170:75–88
Singh RJ (2002) Glutathione: a marker and antioxidant for aging. J Lab Clin Med 140:380–381
Sugimura Y, Yamamoto K (1998) Effect of orally administered reduced- and oxidized-glutathione against acetaminophen-induced liver injury in rats. J Nutr Sci Vitaminol (Tokyo) 44:613–624
Thorpe C, Hoober KL, Raje S, Glynn NM, Burnside J, Turi GK, Coppock DL (2002) Sulfhydryl oxidases: emerging catalysts of protein disulfide bond formation in eukaryotes. Arch Biochem Biophys 405:1–12
Toledano MB, Delaunay-Moisan A, Outten CE, Igbaria A (2013) Functions and cellular compartmentation of the thioredoxin and glutathione pathways in yeast. Antioxid Redox Signal 18:1699–1711
Vartanyan LS, Gurevich S, Kozachenko AI, Nagler LG, Lozovskaya EL, Burlakova EB (2000) Changes in superoxide production rate and in superoxide dismutase and glutathione peroxidase activities in subcellular organelles in mouse liver under exposure to low doses of low-intensity radiation. Biochem Mosc 65:442–446
Yamada R, Taniguchi N, Tanaka T, Ogino C, Fukuda H, Kondo A (2010) Cocktail delta-integration: a novel method to construct cellulolytic enzyme expression ratio-optimized yeast strains. Microb Cell Factories 9:32
Yoshida K, Hariki T, Inoue H, Nakamura T (2002) External skin preparation for whitening. JP Patent 2, 002, 284, 664
Acknowledgments
We are grateful to Dr. J. Ishii (Organization of Advanced Science and Technology, Kobe University) for providing us with the pGK plasmid series. We also thank Dr. M. Mochizuki for technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 134 kb)
Rights and permissions
About this article
Cite this article
Hara, K.Y., Aoki, N., Kobayashi, J. et al. Improvement of oxidized glutathione fermentation by thiol redox metabolism engineering in Saccharomyces cerevisiae . Appl Microbiol Biotechnol 99, 9771–9778 (2015). https://doi.org/10.1007/s00253-015-6847-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6847-z