Abstract
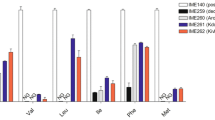
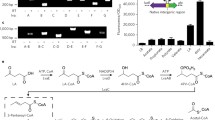
2-Ketoisovalerate is an important cellular intermediate for the synthesis of branched-chain amino acids as well as other important molecules, such as pantothenate, coenzyme A, and glucosinolate. This ketoacid can also serve as a precursor molecule for the production of biofuels, pharmaceutical agents, and flavor agents in engineered organisms, such as the betaproteobacterium Ralstonia eutropha. The biosynthesis of 2-ketoisovalerate from pyruvate is carried out by three enzymes: acetohydroxyacid synthase (AHAS, encoded by ilvBH), acetohydroxyacid isomeroreductase (AHAIR, encoded by ilvC), and dihydroxyacid dehydratase (DHAD, encoded by ilvD). In this study, enzymatic activities and kinetic parameters were determined for each of the three R. eutropha enzymes as heterologously purified proteins. AHAS, which serves as a gatekeeper for the biosynthesis of all three branched-chain amino acids, demonstrated the tightest regulation through feedback inhibition by l-valine (IC50 = 1.2 mM), l-isoleucine (IC50 = 2.3 mM), and l-leucine (IC50 = 5.4 mM). Intermediates in the valine biosynthesis pathway also exhibit feedback inhibitory control of the AHAS enzyme. In addition, AHAS has a very weak affinity for pyruvate (KM = 10.5 μM) and is highly selective towards 2-ketobutyrate (R = 140) as a second substrate. AHAIR and DHAD are also inhibited by the branched-chain amino acids, although to a lesser extent when compared to AHAS. Experimental evolution and rational site-directed mutagenesis revealed mutants of the regulatory subunit of AHAS (IlvH) (N11S, T34I, A36V, T104S, N11F, G14E, and N29H), which, when reconstituted with wild-type IlvB, lead to AHAS having reduced valine, leucine, and isoleucine sensitivity. The study of the kinetics and inhibition mechanisms of R. eutropha AHAS, AHAIR, and DHAD has shed light on interactions between these enzymes and the products they produce; it, therefore, can be used to engineer R. eutropha strains with optimal production of 2-ketoisovalerate for value-added materials.





Similar content being viewed by others
References
Bar-Ilan A, Balan V, Tittmann K, Golbik R, Vyazmensky M, Hübner G, Barak Z, Chipman DM (2001) Binding and activation of thiamin diphosphate in acetohydroxyacid synthase. Biochemistry 40(39):11946–11954
Barak Z, Chipman DM (2012) Allosteric regulation in acetohydroxyacid synthases (AHASs)—different structures and kinetic behavior in isozymes in the same organisms. Arch Biochem Biophys 519(2):167–174
Barak Z, Chipman DM, Gollop N (1987) Physiological implications of the specificity of acetohydroxy acid synthase isozymes of enteric bacteria. J Bacteriol 169(8):3750–3756
Bowien B, Kusian B (2002) Genetics and control of CO2 assimilation in the chemoautotroph Ralstonia eutropha. Arch Microbiol 178(2):85–93
Brigham CJ, Gai CS, Lu J, Speth DR, Worden RM, Sinskey AJ (2013) Engineering Ralstonia eutropha for production of isobutanol from CO2, H2, and O2 in advanced biofuels and bioproducts. Springer, New York 1065–1090
Brigham CJ, Speth DR, Rha C, Sinskey AJ (2012a) Whole-genome microarray and gene deletion studies reveal regulation of the polyhydroxyalkanoate production cycle by the stringent response in Ralstonia eutropha H16. Appl Environ Microbiol 78(22):8033–8044
Brigham CJ, Zhila N, Shishatskaya E, Volova TG, Sinskey AJ (2012) Manipulation of Ralstonia eutropha carbon storage pathways to produce useful bio-based products: reprogramming microbial metabolic pathways in subcellular biochemistry. Springer, Netherlands 64:343–366
Budde CF, Mahan AE, Lu J, Rha C, Sinskey AJ (2010) Roles of multiple acetoacetyl coenzyme A reductases in polyhydroxybutyrate biosynthesis in Ralstonia eutropha H16. J Bacteriol 192(20):5319–5328
Chipman D, Barak Z, Schloss JV (1998) Biosynthesis of 2-aceto-2-hydroxy acids: acetolactate synthases and acetohydroxyacid synthases. Biochim Biophys Acta BBA-Protein Struct Mol Enzymol 1385(2):401–419
Chipman DM, Duggleby RG, Tittmann K (2005) Mechanisms of acetohydroxyacid synthases. Curr Opin Chem Biol 9(5):475–481
Chipman DM, Shaanan B (2001) The ACT domain family. Curr Opin Struct Biol 11(6):694–700
Chunduru SK, Mrachko GT, Calvo KC (1989) Mechanism of ketol acid reductoisomerase steady-state analysis and metal ion requirement. Biochemistry 28(2):486–493
Cramm R (2009) Genomic view of energy metabolism in Ralstonia eutropha H16. J Mol Microbiol Biotechnol 16(1–2):38–52
Duggleby RG (2006) Domain relationships in thiamine diphosphate-dependent enzymes. Acc Chem Res 39(8):550–557
Eggeling I, Cordes C, Eggeling L, Sahm H (1987) Regulation of acetohydroxy acid synthase in Corynebacterium glutamicum during fermentation of α-ketobutyrate to l-isoleucine. Appl Microbiol Biotechnol 25(4):346–351
Eggeling L, Morbach S, Sahm H (1997) The fruits of molecular physiology: engineering the l-isoleucine biosynthesis pathway in Corynebacterium glutamicum. J Biotechnol 56(3):167–182
Engel S, Vyazmensky M, Vinogradov M, Berkovich D, Bar-Ilan A, Qimron U, Rosiansky Y, Barak Z, Chipman DM (2004) Role of a conserved arginine in the mechanism of acetohydroxyacid synthase: catalysis of condensation with a specific ketoacid substrate. J Biol Chem 279(23):24803–24812
Eoyang L, Silverman PM (1986) Role of small subunit (IlvN polypeptide) of acetohydroxyacid synthase I from Escherichia coli K-12 in sensitivity of the enzyme to valine inhibition. J Bacteriol 166(3):901–904
Flint DH, Emptage MH (1988) Dihydroxy acid dehydratase from spinach contains a [2Fe-2S] cluster. J Biol Chem 263(8):3558–3564
Flint DH, Smyk-Randall E, Tuminello JF, Draczynska-Lusiak B, Brown OR (1993) The inactivation of dihydroxy-acid dehydratase in Escherichia coli treated with hyperbaric oxygen occurs because of the destruction of its Fe-S cluster, but the enzyme remains in the cell in a form that can be reactivated. J Biol Chem 268(8):25547–25552
Gollop N, Damri B, Chipman DM, Barak Z (1990) Physiological implications of the substrate specificities of acetohydroxy acid synthases from varied organisms. J Bacteriol 172(6):3444–3449
Grousseau E, Lu J, Gorret N, Guillouet SE, Sinskey AJ (2014) Isopropanol production with engineered Cupriavidus necator as bioproduction platform. Appl Microbiol Biotechnol 98(9):4277–4290
Harper AE, Miller RH, Block KP (1984) Branched-chain amino acid metabolism. Annu Rev Nutr 4(1):409–454
Ishizaki A, Tanaka K, Taga N (2001) Microbial production of poly-d-3-hydroxybutyrate from CO2. Appl Microbiol Biotechnol 57(1–2):6–12
Kaplun A, Vyazmensky M, Zherdev Y, Belenky I, Slutzker A, Mendel S, Barak Z, Chipman DM, Shaanan B (2006) Structure of the regulatory subunit of acetohydroxyacid synthase isozyme III from Escherichia coli. J Mol Biol 357(3):951–963
Kohlmann Y, Pohlmann A, Otto A, Becher D, Cramm R, Lütte S, Schwartz E, Hecker M, Friedrich B (2011) Analyses of soluble and membrane proteomes of Ralstonia eutropha H16 reveal major changes in the protein complement in adaptation to lithoautotrophy. J Proteome Res 10(6):2767–2776
Krause FS, Blombach B, Eikmanns BJ (2010) Metabolic engineering of Corynebacterium glutamicum for 2-ketoisovalerate production. Appl Environ Microbiol 76(24):8053–8061
Kyselková M, Janata J, Ságová-Marečková M, Kopecký J (2010) Subunit–subunit interactions are weakened in mutant forms of acetohydroxy acid synthase insensitive to valine inhibition. Arch Microbiol 192(3):195–200
Lan EI, Liao JC (2013) Microbial synthesis of n-butanol, isobutanol, and other higher alcohols from diverse resources. Bioresour Techno Biorefineries 135(1):339–349
Leyval D, Uy D, Delaunay S, Goergen JL, Engasser JM (2003) Characterisation of the enzyme activities involved in the valine biosynthetic pathway in a valine-producing strain of Corynebacterium glutamicum. J Biotechnol 104(1–3):241–252
Li H, Liao JC (2014) A synthetic anhydrotetracycline-controllable gene expression system in Ralstonia eutropha H16. ACS Synth Biol ASAP
Li H, Opgenorth PH, Wernick DG, Rogers S, Wu TY, Higashide W, Malati P, Huo YX, Cho KM, Liao JC (2012) Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335(7067):1596–1596
Lu J, Brigham CJ, Gai CS, Sinskey AJ (2012) Studies on the production of branched-chain alcohols in engineered Ralstonia eutropha. Appl Microbiol Biotechnol 96(1):283–297
Lu J, Brigham CJ, Rha C, Sinskey AJ (2013) Characterization of an extracellular lipase and its chaperone from Ralstonia eutropha H16. Appl Microbiol Biotechnol 97(6):2443–2454
McCourt JA, Duggleby RG (2006) Acetohydroxyacid synthase and its role in the biosynthetic pathway for branched-chain amino acids. Amino Acids 31(2):173–210
Mendel S, Elkayam T, Sella C, Vinogradov V, Vyazmensky M, Chipman DM, Barak Z (2001) Acetohydroxyacid synthase: a proposed structure for regulatory subunits supported by evidence from mutagenesis. J Mol Biol 307(1):465–477
Mendel S, Vinogradov M, Vyazmensky M, Chipman DM, Barak Z (2003) The N-terminal domain of the regulatory subunit is sufficient for complete activation of acetohydroxyacid synthase III from Escherichia coli. J Mol Biol 325(2):275–284
Park JH, Lee SY (2010) Fermentative production of branched chain amino acids: a focus on metabolic engineering. Appl Microbiol Biotechnol 85(3):491–506
Petkowski JJ, Chruszcz M, Zimmerman MD, Zheng H, Skarina T, Onopriyenko O, Cymborowski MT, Koclega KD, Savchenko A, Edwards A, Minor W (2007) Crystal structures of TM0549 and NE1324–two orthologs of E. coli AHAS isozyme III small regulatory subunit. Protein Sci Publ Protein Soc 16(7):1360–1367
Pohlmann A, Fricke WF, Reinecke F, Kusian B, Liesegang H, Cramm R, Eitinger T, Ewering C, Pötter M, Schwartz E, Strittmatter A, Voss I, Gottschalk G, Steinbüchel A, Friedrich B, Bowien B (2006) Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat Biotechnol 24(10):1257–1262
Quandt J, Hynes MF (1993) Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127(1):15–21
Raberg M, Voigt B, Hecker M, Steinbüchel A (2014) A closer look on the polyhydroxybutyrate-(PHB-) negative phenotype of Ralstonia eutropha PHB-4. PloS One 9(5):e95907
Slater KLH (1998) Multiple beta-ketothiolases mediate poly(beta-hydroxyalkanoate) copolymer synthesis in Ralstonia eutropha. J Bacteriol 180(8):1979–87
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Schomburg D, Stephan D (1995) Ketol-acid reductoisomerase. Springer, Berlin. 433–437
Schwartz E, Voigt B, Zühlke D, Pohlmann A, Lenz O, Albrecht D, Schwarze A, Kohlmann Y, Krause C, Hecker M, Friedrich B (2009) A proteomic view of the facultatively chemolithoautotrophic lifestyle of Ralstonia eutropha H16. Proteomics 9(22):5132–5142
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol 1(9):784–791
Slutzker A, Vyazmensky M, Chipman DM, Barak Z (2011) Role of the C-terminal domain of the regulatory subunit of AHAS isozyme III: use of random mutagenesis with in vivo reconstitution (REM-ivrs). Biochim Biophys Acta 1814(3):449–455. doi:10.1016/j.bbapap.2011.01.002
Steinmetz A, Vyazmensky M, Meyer D, Barak ZE, Golbik R, Chipman DM, Tittmann K (2010) Valine 375 and phenylalanine 109 confer affinity and specificity for pyruvate as donor substrate in acetohydroxy acid synthase isozyme II from Escherichia coli. Biochemistry 49(25):5188–5199
Tittmann K, Schröder K, Golbik R, McCourt J, Kaplun A, Duggleby RG, Barak Z, Chipman DM, Hübner G (2004) Electron transfer in acetohydroxy acid synthase as a side reaction of catalysis: implications for the reactivity and partitioning of the carbanion/enamine Form of (α-Hydroxyethyl)thiamin diphosphate in a “nonredox” flavoenzyme. Biochemistry 43(27):8652–8661
Vinogradov V, Vyazmensky M, Engel S, Belenky I, Kaplun A, Kryukov O, Barak Z, Chipman DM (2006) Acetohydroxyacid synthase isozyme I from Escherichia coli has unique catalytic and regulatory properties. Biochim Biophys Acta 1760(3):356–363
Vollbrecht D, El Nawawy MA, Schlegel HG (1978) Excretion of metabolites by hydrogen bacteria. I. Autotrophic and heterotrophic fermentations. Eur J Appl Microbiol Biotechnol 6:145–155
Vollbrecht D, Schlegel HG (1978) Excretion of metabolites by hydrogen bacteria. II. Influences of aeration, pH, temperature, and age of cells. Eur J Appl Microbiol Biotechnol 6:157–166
Vollbrecht D, Schlegel HG (1979) Excretion of metabolites by hydrogen bacteria. III. D(-)-3-hydroxybutanoate. Eur J Appl Microbiol Biotechnol 7:259–266
Vyazmensky M, Sella C, Barak Z, Chipman DM (1996) Isolation and characterization of subunits of acetohydroxy acid synthase isozyme III and reconstitution of the holoenzyme. Biochemistry 35(32):10339–10346
Vyazmensky M, Steinmetz A, Meyer D, Golbik R, Barak Z, Tittmann K, Chipman DM (2011) Significant catalytic roles for Glu47 and Gln 110 in all four of the C-C bond-making and -breaking steps of the reactions of acetohydroxyacid synthase II. Biochemistry 50(15):3250–3260
Vyazmensky M, Zherdev Y, Slutzker A, Belenky I, Kryukov O, Barak Z, Chipman DM (2009) Interactions between large and small subunits of different acetohydroxyacid synthase isozymes of Escherichia coli. Biochemistry 48(36):8731–8737
Westerfield WW (1945) A colorimetric determination of blood acetoin. J Biol Chem 161(1):495–502
Wilde E (1962) Untersuchungen ϋber wachstum und speicherstoffsynthese von hydroenomonas. Archiv Fϋr Mikrobiologie 43(2):109–137
York GM, Stubbe J, Sinskey AJ (2001) New insight into the role of the PhaP phasin of Ralstonia eutropha in promoting synthesis of polyhydroxybutyrate. J Bacteriol 183(7):2394–2397
Zor T, Selinger Z (1996) Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem 236(2):302–308
Acknowledgments
The authors thank Dr. Claudia Santos Gai for helpful discussions, Mr. John W. Quimby for critical review of this manuscript, and Mr. Caio Alves for assistance with R. eutropha experimental evolution experiment. This work is funded by the U.S. Department of Energy, Advanced Research Projects Agency-Energy (ARPA-E). JL is supported by the Malaysian Palm Oil Board.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 375 kb)
Rights and permissions
About this article
Cite this article
Lu, J., Brigham, C.J., Plassmeier, J.K. et al. Characterization and modification of enzymes in the 2-ketoisovalerate biosynthesis pathway of Ralstonia eutropha H16. Appl Microbiol Biotechnol 99, 761–774 (2015). https://doi.org/10.1007/s00253-014-5965-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5965-3