Abstract
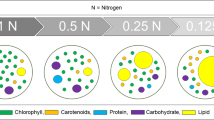
Chlorella vulgaris accumulates lipid under nitrogen limitation, but at the expense of biomass productivity. Due to this tradeoff, improved lipid productivity may be compromised, despite higher lipid content. To determine the optimal degree of nitrogen limitation for lipid productivity, batch cultures of C. vulgaris were grown at different nitrate concentrations. The growth rate, lipid content, lipid productivity and biochemical and elemental composition of the cultures were monitored for 20 days. A starting nitrate concentration of 170 mg L−1 provided the optimal tradeoff between biomass and lipid production under the experimental conditions. Volumetric lipid yield (in milligram lipid per liter algal culture) was more than double that under nitrogen-replete conditions. Interpolation of the data indicated that the highest volumetric lipid concentration and lipid productivity would occur at nitrate concentrations of 305 and 241 mg L−1, respectively. There was a strong correlation between the nitrogen content of the cells and the pigment, protein and lipid content, as well as biomass and lipid productivity. Knowledge of the relationships between cell nitrogen content, growth, and cell composition assists in the prediction of the nitrogen regime required for optimal productivity in batch or continuous culture. In addition to enhancing lipid productivity, nitrogen limitation improves the lipid profile for biodiesel production and reduces the requirement for nitrogen fertilizers, resulting in cost and energy savings and a reduction in the environmental burden of the process.






Similar content being viewed by others
References
Apt KE, Behrens PW (1999) Commercial developments in microalgal biotechnology. J Phycol 2:215–226
Bold HC (1949) The morphology of Chlamydomonas chlamydogama sp. nov. Bull Torrey Bot Club 76:101–108
Borowitzka MA (1992) Algal biotechnology products and processes—matching science and economics. J Appl Phycol 4:267–279
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgal strains. Bioresour Technol 124:217–226
Chelf P (1990) Environmental control of lipid and biomass production in two diatom species. J Appl Phycol 2:121–129
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 6:1146–1151
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Griffiths MJ, van Hille RP, Harrison STL (2010) Selection of direct transesterification as the preferred method for assay of fatty acid content of microalgae. Lipids 45(11):1053–1060
Griffiths MJ, Garcin C, van Hille RP, Harrison STL (2011) Interference by pigment in the estimation of microalgal biomass concentration by optical density. J Microbiol Methods 85(2):119–123
Griffiths MJ, van Hille RP, Harrison STL (2012) Lipid productivity, settling potential and fatty acid profile of eleven microalgal species grown under nitrogen replete and limited conditions. J Appl Phycol 24(5):989–1001
Hsieh C, Wu W (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 17:3921–3926
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639
Illman A, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol 27(8):631–635
Klok AJ, Martens DE, Wijffels RH, Lamers PP (2013) Simultaneous growth and neutral lipid accumulation in microalgae. Bioresour Technol 134:233–243
Lacour T, Sciandra A, Talec A, Mayzaud P, Bernard O (2012) Neutral lipid and carbohydrate productivities as a response to nitrogen status in Isochrysis sp. (T.iso; Haptophyceae): starvation versus limitation. J Phycol 656:647–656
Langley N, Harrison STL, van Hille RP (2012) A critical evaluation of CO2 supplementation to algal systems by direct injection. Biochem Eng J 68:70–75
Lardon L, HeÌlias A, Sialve B, Steyer JP, Bernard O (2009) Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol 17:6475–6481
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 4:629–636
Lv J-M, Cheng L-H, Xu X-H, Zhang L, Chen H-L (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101(17):6797–6804
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 1:217–232
Pedro AS, Gonzalez-Lopez CV, Acién FG, Molina-Grima E (2013) Marine microalgae selection and culture conditions optimization for biodiesel production. Bioresour Technol 134:353–361
Piorreck M, Baasch K, Pohl P (1984) Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 2:207–216
Pruvost J, Van Vooren G, Cogne G, Legrand J (2009) Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresour Technol 100(23):5988–5995
Rausch T (1981) The estimation of micro-algal protein content and its meaning to the evaluation of algal biomass I. Comparison of methods for extracting protein. Hydrobiologia 78(3):237–251
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 1:100–112
Roessler P (1990) Environmental control of glycerolipid metabolism in microalgae: commercial implications and future research directions. J Phycol 26:393–399
Sheehan J, Dunahay T, Benemann JR, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program: biodiesel from algae. Closeout report. National Renewable Energy Lab, Department of Energy, Golden, Colorado, U.S.A. Report number NREL/TP-580-24190, July 1998
Shifrin NS, Chisholm SW (1981) Phytoplankton lipids: interspecific differences and effects of nitrate, silicate and light–dark cycles. J Phycol 17:374–384
Stephenson AL, Dennis JS, Howe CJ, Scott SA, Smith AG (2010) Influence of nitrogen-limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biogeosciences 1:47–58
Su C, Chien L, Gomes J (2011) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol 23:903–908
Tsukahara K, Sawayama S (2005) Liquid fuel production using microalgae. J Jpn Pet Inst 5:251–259
Walker JM (1994) Methods in molecular biology, vol. 32: basic protein and peptide protocols. Humana Press, New Jersey
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Widjaja A, Chien CC, Ju YH (2009) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem Eng 40(1):13–20
Acknowledgments
This work is based upon research supported by the South African National Energy Development Institute (SANEDI), the South African Research Chairs Initiative (SARChI) of the Department of Science and Technology, the National Research Foundation (NRF), and the Technology Innovation Agency (TIA). The financial assistance of these organizations is hereby acknowledged. Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to SANEDI, SARChI, TIA, or the NRF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Griffiths, M.J., van Hille, R.P. & Harrison, S.T.L. The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris . Appl Microbiol Biotechnol 98, 2345–2356 (2014). https://doi.org/10.1007/s00253-013-5442-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5442-4