Abstract
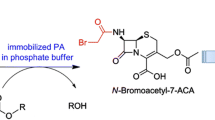
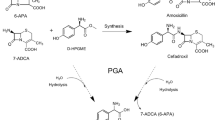
Penicillin G acylase from Achromobacter sp. (NPGA) was studied in the enzymatic synthesis of β-lactam antibiotics by kinetically controlled N-acylation. When compared with penicillin acylase of Escherichia coli (PGA), the NPGA was significantly more efficient at syntheses of ampicillin and amoxicillin (higher S/H ratio and product accumulation) in the whole range of substrate concentrations. The degree of conversion of 6-aminopenicillanic acid to amoxicillin and ampicillin (160 mM 6-APA, 350 mM acyl donor methylester⋅HCl, pH 6.3, 25 °C, reaction time of 200 min) with immobilized NPGA equaled 96.9 % and 91.1 %, respectively. The enzyme was highly thermostable with maximum activity at 60 °C (pH 8.0) and 65 °C (pH 6.0). Activity half-life at 60 °C (pH 8.0) and at 60 °C (pH 6.0) was 24 min and 6.9 h, respectively. Immobilized NPGA exhibited long operational stability with half-life of about 2,000 cycles for synthesis of amoxicillin at conversion conditions used in large-scale processes (230 mM 6-APA, 340 mM d-4-hydroxyphenylglycine methylester⋅HCl, 27.5 °C, pH 6.25). We discuss our results with literature data available for related penicillin acylases in terms of their industrial potential.





Similar content being viewed by others
References
Alkema WBL, Dijkhuis AJ, deVries E, Janssen DB (2002) The role of hydrophobic active-site residues in substrate specificity and acyl transfer activity of penicillin acylase. Eur J Biochem 269:2093–2100
Bruggink A, Roos EC, de Vroom E (1998) Penicillin acylase in the industrial production of β-lactam antibiotics. Org Process Res Dev 2:128–133
Cai G, Zhu S, Yang S, Zhao G, Jiang W (2004) Cloning, overexpression, and characterization of a novel thermostable penicillin G acylase from Achromobacter xylosoxidans: probing the molecular basis for its high thermostability. Appl Environ Microbiol 70:2764–2770
Chandel AK, Rao LV, Narasu ML, Singh OV (2008) The realm of penicillin G acylase in β-lactam antibiotics. Enzyme Microb Technol 42:199–207
Cheng T, Chen M, Zheng H, Wang J, Yang S, Jiang W (2006) Expression and purification of penicillin G acylase enzymes from four different micro-organisms, and a comparative evaluation of their synthesis/hydrolysis ratios for cephalexin. Protein Expr Purif 46:107–113
Datla A, Rajasekar VW, Kyslík P, Bečka S, Krishnakant AT, Yogesh ZS, Nikunj K (2011) Process for the preparation of penicillin or cephalosporin antibiotics. EP 2173892
Diender MB, Straathof AJJ, van der Wielen LAM, Ras C, Heijden JJ (1998) Feasibility of the thermodynamically controlled synthesis of amoxicillin. J Mol Catal B Enzym 5:249–253
Diender MB, Straathof AJJ, van der Does T, Zomerdijk M, Heijden JJ (2000) Course of pH during the formation of amoxicillin by a suspension-to-suspension reaction. Enzyme Microb Technol 27:576–582
Duggleby HJ, Tolley SP, Hill CP, Dodson EJ, Dodson G, Moody PC (1995) Penicillin acylase has a single-amino-acid catalytic centre. Nature 373:264–268
Gabor EM, de Vries EJ, Janssen DB (2005) A novel penicillin acylase from the environmental gene pool with improved synthetic properties. Enzyme Microb Technol 36:182–190
Guranda DT, Volovik TS, Švedas VK (2004) pH stability of penicillin acylase from Escherichia coli. Biochem Mosc 69:1386–1390
Hernández-Jústiz O, Terreni M, Pagani G, García JL, Guisán JM, Fernández-Lafuente R (1999) Evaluation of different enzymes as catalysts for the production of β-lactam antibiotics following a kinetically controlled strategy. Enzyme Microb Technol 25:336–343
Ignatova Z, Wischnewski F, Notbohm H, Kasche V (2005) Prosequence and Ca2+-binding: implications for folding and maturation of Ntn-hydrolase penicillin amidase from E. coli. J Mol Biol 348:999–1014
Illanes A, Wilson L, Corrotea O, Tavernini L, Zamorano F, Aguirre C (2007) Synthesis of cephalexin with immobilized penicillin acylase at very high substrate concentrations in fully aqueous medium. J Mol Catal B Enzym 47:72–78
Jager SAW, Jekel PA, Janssen DB (2007) Hybrid penicillin acylases with improved properties for synthesis of β-lactam antibiotics. Enzyme Microb Technol 40:1335–1344
Kasche V, Galunsky B, Ignatova Z (2003) Fragments of pro-peptide activate mature penicillin amidase of Alcaligenes faecalis. Eur J Biochem 270:4721–4728
Kutzbach C, Rauenbusch E (1974) Preparation and general properties of crystalline penicillin acylase from Escherichia coli ATCC 11105. H-S Z Physiol Chem 354:45–53
Kyslík P, Štěpánek V, Hollerová L, Bečka S, Vyasarayani WR, Datla A, Plháčkova K, Maršálek J (2011) DNA sequence encoding penicillin acylase, novel recombinant recombinant DNA constructs and recombinant microorganisms. US 8039604
Ospina S, Barzana E, Ramírez OT, López-Munguía A (1996) Effect of pH in the synthesis of ampicillin by penicillin acylase. Enzyme Microb Technol 19:462–469
Plháčková K, Bečka S, Škrob F, Kyslík P (2003) Isolation and characterization of a new strain of Achromobacter sp. with β-lactam antibiotic acylase activity. Appl Microbiol Biotechnol 62:507–516
Ribeiro MPA, Ferreira ALO, Giordano RLC, Giordano RC (2005) Selectivity of enzymatic synthesis of ampicillin by E. coli PGA in the presence of high concentrations of substrates. J Mol Catal B Enzym 33:81–86
Schroën CGPH, Nierstrasz VA, Kroon PJ, Bosma R, Janssen AEM, Beeftink HH, Tramper J (1999) Thermodynamically controlled synthesis of β-lactam antibiotics. Equilibrium concentrations and side-chain properties. Enzyme Microb Technol 24:498–506
Škrob F, Bečka S, Plháčková K, Fotopolusová V, Kyslík P (2003) Novel penicillin G acylase from Achromobacter sp. CCM 4824. Enzyme Microb Technol 32:738–744
Sobotková L, Štěpánek V, Plháčková K, Kyslík P (1996) Development of a high-expression system for penicillin G acylase based on the recombinant Escherichia coli strain RE3(pKA18). Enzyme Microb Technol 19:389–397
Švedas V, Guranda D, van Langen L, van Rantwijk F, Sheldon R (1997) Kinetic study of penicillin acylase from Alcaligenes faecalis. FEBS Lett 417:414–418
Youshko MI, van Langen LM, de Vroom E, Moody HM, van Rantwijk F, Sheldon RA, Švedas VK (2000) Penicillin acylase-catalyzed synthesis of ampicillin in “aqueous solution–precipitate” systems. High substrate concentration and supersaturation effect. J Mol Catal B Enzym 10:509–515
Youshko MI, van Langen LM, de Vroom E, van Rantwijk F, Sheldon RA, Švedas VK (2001) Highly efficient synthesis of ampicillin in an “aqueous solution–precipitate” system: repetitive addition of substrates in a semicontinuous process. Biotechnol Bioeng 73:426–430
Youshko MI, Chilov GG, Shcherbakova TA, Švedas VK (2002) Quantitative characterization of the nucleophile reactivity in penicillin acylase-catalyzed acyl transfer reactions. Biochim Biophys Acta 1599:134–140
Acknowledgments
This research was supported by Fermenta Biotech Ltd. and by long-term research development project RVO 61388971 of the Institute of Microbiology ASCR, v.v.i.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bečka, S., Štěpánek, V., Vyasarayani, R.W. et al. Penicillin G acylase from Achromobacter sp. CCM 4824. Appl Microbiol Biotechnol 98, 1195–1203 (2014). https://doi.org/10.1007/s00253-013-4945-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4945-3