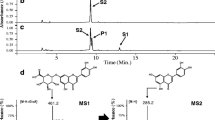
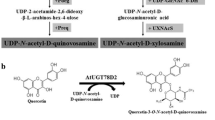
Abstract
Regioselective glycosylation of flavonoids cannot be easily achieved due to the presence of several hydroxyl groups in flavonoids. This hurdle could be overcome by employing uridine diphosphate-dependent glycosyltransferases (UGTs), which use nucleotide sugars as sugar donors and diverse compounds including flavonoids as sugar acceptors. Quercetin rhamnosides contain antiviral activity. Two quercetin diglycosides, quercetin 3-O-glucoside-7-O-rhamnoside and quercetin 3,7-O-bisrhamnoside, were synthesized using Escherichia coli expressing two UGTs. For the synthesis of quercetin 3-O-glucoside-7-O-rhamnoside, AtUGT78D2, which transfers glucose from UDP-glucose to the 3-hydroxyl group of quercetin, and AtUGT89C1, which transfers rhamnose from UDP-rhamnose to the 7-hydroxyl group of quercetin 3-O-glucoside, were transformed into E. coli. Using this approach, 67 mg/L of quercetin 3-O-glucoside-7-O-rhamnoside was synthesized. For the synthesis of quercetin 3,7-O-bisrhamnoside, AtUGT78D1, which transfers rhamnose to the 3-hydroxy group of quercetin, and AtUGT89C1 were used. The RHM2 gene from Arabidopsis thaliana was coexpressed to supply the sugar donor, UDP-rhamnose. E. coli expressing AtUGT78D1, AtUGT89C1, and RHM2 was used to obtain 67.4 mg/L of quercetin 3,7-O-bisrhamnoside.





Similar content being viewed by others
References
Bowles D, Isayenkova J, Lim E-K, Poppenberger B (2005) Glycosyltransferase: mangers of small molecules. Curr Opin Plant Biol 8:254–263
Bowles D, Lim E-K, Poppenberger B, Vaistij FE (2006) Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol 57:567–597
Choi HJ, Song JH, Park KS, Kwon DH (2009a) Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur J Pharm Sci 3:329–333
Choi HJ, Kim JH, Lee CH, Ahn YJ, Song JH, Baek SH, Kwon DH (2009b) Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antivir Res 81:77–81
Dong C, Beis K, Giraud MF, Blankenfeldt W, Allard S, Major LL, Kerr ID, Whitfield C, Naismith JH (2003) A structural perspective on the enzymes that convert dTDP-D-glucose into dTDP-L-rhamnose. Biochem Soc Trans 31:532–536
Du Y, Wei G, Linhardt RJ (2004) Total synthesis of quercetin 3-sophorotrioside. J Org Chem 69:2206–2209
Graham TL (1991) Flavonoid and isoflavonoid distribution in developing soybean seedling tissues and in seed and root exudates. Plant Physiol 95:594–603
Harper A, Bar-Peled M (2002) Biosynthesis of UDP-xylose. Cloning and characterization of a novel Arabidopsis gene family, UXS, encoding soluble and putative membrane-bound UDP-glucuronic acid decarobylase isoforms. Plant Physiol 130:2188–2198
Jones P, Messner B, Nakajima J-I, Schäffner AR, Saito K (2003) UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. J Biol Chem 278:43910–43918
Kim B-G, Jung NR, Joe EJ, Hur H-G, Lim Y, Chong Y, Ahn J-H (2010) Bacterial synthesis of a flavonoid deoxyaminosugar conjugate in Escherichia coli expressing a glycosyltransferase of Arabidopsis thaliana. ChemBioChem 11:2389–2392
Kim B-G, Sung SH, Ahn J-H (2012a) Biological synthesis of quercetin 3-O-N-acetylglucosamine conjugate using engineered Escherichia coli expressing UGT78D2. Appl Microbiol Biotechnol 93:2447–2453
Kim BG, Kim HJ, Ahn J-H (2012b) Production of bioactive flavonol rhamnosides by expression of plant genes in Escherichia coli. J Agric Food Chem 60:11143–11148
Ko JH, Kim BG, Ahn J-H (2006) Glycosylation of flavonoids with a glycosyltransferase from Bacillus cereus. FEMS Microbiol Lett 258:263–268
Ku KM, Choi JN, Kim J, Kim JK, Yoo LG, Lee SJ, Hong Y-S, Lee CH (2010) Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J Agric Food Chem 58:418–426
Li M, Han X, Yu B (2003) Facile synthesis of flavonoid 7-O-glycosides. J Org Chem 68:6842–6845
Lim E-K, Ashford DA, Hou B, Jackson RG, Bowles DJ (2004) Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetin glucosides. Biotech Bioeng 87:623–631
Oka T, Nemoto T, Jigami Y (2007) Functional analysis of Arabidopsis thaliana RHM2/MUM4, a multidomain protein involved in UDP-D-glucose to UDP-L-rhamnose conversion. J Biol Chem 282:5389–5403
Ono E, Homma Y, Horikawa M, Kunikane-Doi S, Imai H, Takahashi S, Kawai Y, Ishiguro M, Fukui Y, Nakayama T (2010) Functional differentiation of the glycosyltransferases that contribute to the chemical diversity of bioactive flavonol glycosides in grapevines (Vitis vinifera). Plant Cell 22:2856–2871
Osmani SA, Bak S, Mϕller BL (2009) Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry 70:325–347
Paquette S, Mϕller BL, Bak S (2003) On the origin of family 1 plant glycosyltransferase. Phytochemistry 62:399–413
Song JH, Shim JK, Choi HJ (2011) Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virol J 8:460
Sung SH, Kim BG, Ahn J-H (2011) Optimization of rhamnetin production in Escherichia coli. J Micorbiol Biotechnol 21:845–857
Thibodeaux CJ, Melanҫon CE, H-w L (2007) Unusual sugar biosynthesis and natural product glycodiversification. Nature 446:1008–1016
Thorson JS, Hosted TJ Jr, Jiang J, Biggins JB, Ahlert J (2001) Natures carbohydrate chemists the enzymatic glycosylation of bioactive bacterial metabolites. Curr Org Chem 5:139–167
Veit M, Pauli GF (1999) Major flavonoids from Arabidopsis thaliana leaves. J Nat Prod 62:1301–1303
Vogt T, Jones P (2000) Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends Plant Sci 5:380–386
Williams GJ, Gantt RW, Thorson JS (2008) The impact of enzyme engineering upon natural product glucodiversification. Curr Opin Chem Biol 12:556–564
Yonekura-Sakakibara K, Tohge T, Niida R, Saito K (2007) Identification of a flavonol 7-O-rhamnosyltransferase gene determining flavonoid pattern in Arabidopsis by transcriptome coexpression analysis and reverse genetics. J Biol Chem 282:14931–14941
Acknowledgments
This work was supported by a grant from Next-Generation BioGreen 21 Program (PJ00948301), Rural Development Administration, Republic of Korea and by the Priority Research Centers Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012–0006686).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H.J., Kim, BG. & Ahn, JH. Regioselective synthesis of flavonoid bisglycosides using Escherichia coli harboring two glycosyltransferases. Appl Microbiol Biotechnol 97, 5275–5282 (2013). https://doi.org/10.1007/s00253-013-4844-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4844-7