Abstract
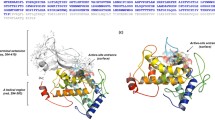
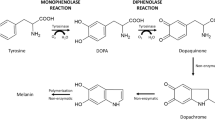
Tyrosinase is a member of the type 3 copper enzyme family involved in the production of melanin in a wide range of organisms. The ability of tyrosinases to convert monophenols into diphenols has stimulated studies regarding the production of substituted catechols, important intermediates for the synthesis of pharmaceuticals, agrochemicals, polymerization inhibitors, and antioxidants. Despite its enormous potential, the use of tyrosinases for catechol synthesis has been limited due to the low monophenolase/diphenolase activity ratio. In the presence of two water miscible ionic liquids, [BMIM][BF4] and ethylammonium nitrate, the selectivity of a tyrosinase from Bacillus megaterium (TyrBm) was altered, and the ratio of monophenolase/diphenolase activity increased by up to 5-fold. Furthermore, the addition of sodium dodecyl sulphate (SDS) at levels of 2–50 mM increased the activity of TyrBm by 2-fold towards the natural substrates l-tyrosine and l-Dopa and 15- to 20-fold towards the non-native phenol and catechol. The R209H tyrosinase variant we previously identified as having a preferential ratio of monophenolase/diphenolase activity was shown to have a 45-fold increase in activity towards phenol in the presence of SDS. We propose that the effect of SDS on the ability of tyrosinase to convert non-natural substrates is due to the interaction of surfactant molecules with residues located at the entrance to the active site, as visualized by the newly determined crystal structure of TyrBm in the presence of SDS. The effect of SDS on R209 may enable less polar substrates such as phenol and catechol, to penetrate more efficiently into the enzyme catalytic pocket.






Similar content being viewed by others
References
Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr Sect D Biol Crystallogr 66: 213–221.
Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr Sect D Biol Crystallogr 54: 905–921.
Burton SG (2003) Oxidizing enzymes as biocatalysts. Trends Biotechnol 21: 543–549.
Claus H, Decker H (2006) Bacterial tyrosinases. Syst Appl Microbiol 29: 3–14.
Cong Y, Zhang Q, Woolford D, Schweikardt T, Khant H, Dougherty M, Ludtke SJ, Chiu W, Decker H (2009) Structural mechanism of SDS-induced enzyme activity of scorpion hemocyanin revealed by electron cryomicroscopy. Structure 17: 749–758.
Decker H, Tuczek F (2000) Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism. Trends Biochem Sci 25: 392–397.
Decker H, Schweikardt T, Nillius D, Salzbrunn U, Jaenicke E, Tuczek F (2007) Similar enzyme activation and catalysis in hemocyanins and tyrosinases. Gene 398: 183–191.
Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr Sect D Biol Crystallogr 60: 2126–2132.
Gandia-Herrero F, Jimenez-Atienzar M, Cabanes J, Garcia-Carmona F, Escribano J (2005) Differential activation of a latent polyphenol oxidase mediated by sodium dodecyl sulfate. J Agric Food Chem 53: 6825–6830.
Halaouli S, Asther M, Sigoillot JC, Hamdi M, Lomascolo. A (2006) Fungal tyrosinases: new prospects in molecular characteristics, bioengineering and biotechnological applications. J Appl Microbiol 100: 219–232.
Hernandez-Romero D, Sanchez-Amat A, Solano F (2006) A tyrosinase with an abnormally high tyrosine hydroxylase/dopa oxidase ratio. FEBS J 273: 257–270.
Itoh S, Fukuzumi S (2007) Monooxygenase activity of type 3 copper proteins. Acc Chem Res 40: 592–600.
Karbassi F, Haghbeen K, Saboury AA, Rezaei-Tavirani M, Ranjbar B (2004) Calorimetric, spectrophotometric and circular dichroism studies on the impact of sodium dodecyl sulfate on the mushroom tyrosinase structure. Biologia 59: 319–326.
Kawamura-Konishi Y, Tsuji M, Hatana S, Asanuma M, Kakuta D, Kawano T, Mukouyama EB, Goto H, Suzuki H (2007) Purification, characterization, and molecular cloning of tyrosinase from Pholiota nameko. Biosci, Biotechnol, Biochem 71: 1752–1760.
Klabunde T, Eicken C, Sacchettini JC, Krebs B (1998) Crystal structure of plant catechol oxidase containing a dicopper center. Nat Struct Biol 5: 1084–1090.
Leslie AGW (1992) Joint CCP4 + ESF-EAMCB Newsletter on protein crystallography: No. 26.
Lopez-Serrano D, Sanchez-Amat A, Solano F (2002) Cloning and molecular characterization of a SDS-activated tyrosinase from Marinomonas mediterranea. Pigment Cell Res 15: 104–111.
Martinez MV, Whitaker JR (1995) The biochemistry and control of enzymatic browning. Trends Food Sci Technol 6: 195–200.
McCoy AJ (2007) Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr Sect D Biol Crystallogr 63: 32–41.
Moore BM, Flurkey WH (1990) Sodium dodecyl sulfate activation of a plant polyphenoloxidase. Effect of sodium dodecyl sulfate on enzymatic and physical characteristics of purified broad bean polyphenoloxidase. J Biol Chem 265: 4982–4988.
Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr Sect D Biol Crystallogr 53: 240–255.
Neeley E, Fritch G, Fuller A, Wolfe J, Wright J, Flurkey W (2009) Variations in IC50 values with purity of mushroom tyrosinase. Int J Mol Sci 10: 3811–3823.
Nillius D, Jaenicke E, Decker H (2008) Switch between tyrosinase and catecholoxidase activity of scorpion hemocyanin by allosteric effectors. FEBS Lett 582: 749–754.
Nolan LC, O'Connor KE (2007) Use of Pseudomonas mendocina, or recombinant Escherichia coli cells expressing toluene-4-monooxygenase, and a cell-free tyrosinase for the synthesis of 4-fluorocatechol from fluorobenzene. Biotechnol Lett 29: 1045–1050.
Olivares C, Solano F (2009) New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigm Cell Melanoma R 22: 750–760.
Olivares C, Garcia-Borron JC, Solano F (2002) Identification of active site residues involved in metal cofactor binding and stereospecific substrate recognition in mammalian tyrosinase. Implications to the catalytic cycle. Biochemistry 41: 679–686.
Rodriguez-Lopez JN, Escribano J, Garciacanovas F (1994) A continuous spectrophotometric method for the determination of monophenolase activity of tyrosinase using 3-methyl-2-benzothiazolinone hydrazone. Anal Biochem 216: 205–212.
Saeidian S, Keyhani E, Keyhani J (2007) Effect of ionic detergents, nonionic detergents, and chaotropic agents on polyphenol oxidase activity from dormant saffron (Crocus stivus L.) corms. J Agric Food Chem 55: 3713–3719.
Selinheimo E, NiEidhin D, Steffensen C, Nielsen J, Lomascolo A, Halaouli S, Record E, O'Beirne D, Buchert J, Kruus K (2007) Comparison of the characteristics of fungal and plant tyrosinases. J Biotechnol 130: 471–480.
Sendovski M, Kanteev M, Shuster V, Adir N, Fishman A (2010) Primary x-ray crystallographic analysis of a bacterial tyrosinase from Bacillus megaterium. Acta Crystallogr Sect F Struct Biol Crystallogr 66: 1101–1103.
Sendovski M, Kanteev M, Shuster Ben-Yosef V, Adir N, Fishman A (2011) First structures of an active bacterial tyrosinase reveal copper plasticity. J Mol Biol 405: 227–237.
Shuster Ben-Yosef V, Sendovski M, Fishman A (2010) Directed evolution of tyrosinase for enhanced monophenolase/diphenolase activity ratio. Enzyme Microb Technol 47: 372–376.
Shuster V, Fishman A (2009) Isolation, cloning and characterization of a tyrosinase with improved activity in organic solvents from Bacillus megaterium. J Mol Microbiol Biotechnol 17: 188–200.
Skubak P, Murshudov GN, Pannu NS (2004) Direct incorporation of experimental phase information in model refinement. Acta Crystallogr Sect F Struct Biol Crystallogr 60: 2196–2201.
van Holde KE, Miller KI, Decker H (2001) Hemocyanins and invertebrate evolution. J Biol Chem 276: 15563–15566.
Wang G, Aazaz A, Peng Z, Shen P (2000) Cloning and overexpression of a tyrosinase gene mel from Pseudomonas maltophila. FEMS Microbiol Lett 185: 23–27.
Xiang J, Fan J-B, Chen N, Chen J, Liang Y (2006) Interaction of cellulase with sodium dodecyl sulfate at critical micelle concentration level. Colloids Surf B Biointerfaces 49: 175–180.
Yang Z (2009) Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysis. J Biotechnol 144: 12–22.
Yang Z, Pan W (2005) Ionic liquids: green solvents for nonaqueous biocatalysis. Enzyme Microb Technol 37: 19–28.
Yang Z, Yue YJ, Xing M (2008) Tyrosinase activity in ionic liquids. Biotechnol Lett 30: 153–158.
Yang Z, Yue YJ, Huang WC, Zhuang XM, Chen ZT, Xing M (2009) Importance of the ionic nature of ionic liquids in affecting enzyme performance. J Biochem (Tokyo) 145: 355–364.
Zhou J, Shi P, Zhang R, Huang H, Meng K, Yang P, Yao B (2011) Symbiotic Streptomyces sp. TN119 GH 11 xylanase: a new pH-stable, protease- and SDS-resistant xylanase. J Ind Microbiol Biotechnol 38: 523–530.
Acknowledgments
This work was supported by the Israel Science Foundation founded by the Israel Academy of Sciences and Humanities, grant number 193/11. We gratefully thank the staff of the ESRF (beamline ID23-1) for provision of synchrotron radiation facilities and assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goldfeder, M., Egozy, M., Shuster Ben-Yosef, V. et al. Changes in tyrosinase specificity by ionic liquids and sodium dodecyl sulfate. Appl Microbiol Biotechnol 97, 1953–1961 (2013). https://doi.org/10.1007/s00253-012-4050-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4050-z