Abstract
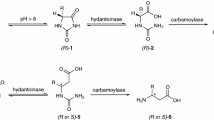
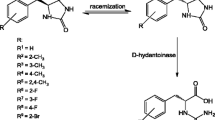
In this study, we investigated the possibility of using a modified hydantoinase process for the production of optically pure β-amino acids. Two aryl-substituted dihydropyrimidines d,l-6-phenyl-5,6-dihydrouracil (PheDU) and para-chloro-d,l-6-phenyl-5,6-dihydrouracil (pClPheDU) were synthesized. Hydrolysis of these novel substrates to the corresponding N-carbamoyl-β-amino acids by three recombinant d-hydantoinases and several bacterial strains was tested. All applied recombinant d-hydantoinases and eight bacterial isolates catalyzed the conversion of PheDU to N-carbamoyl-β-phenylalanine (NCβPhe). Some of these biocatalysts showed an enantioselectivity for either the d- or the l-PheDU enantiomer. The second dihydropyrimidinase substrate pClPheDU was hydrolyzed by all three recombinant d-hydantoinases and six of the wild-type strains. To our knowledge, this is the first dihydropyrimidinase activity reported with this aryl-substituted dihydropyrimidine. For selected biocatalysts, hydantoinase activity towards aryl-substituted hydantoins was demonstrated as well. However, none of the bacterial strains tested so far exhibited any carbamoylase activity towards NCβPhe.



Similar content being viewed by others
References
Andújar-Sánchez M, Las Heras-Vázquez FJ, Clemente-Jiménez JM, Martínez-Rodríguez S, Camara-Artigas A, Rodríguez-Vico F, Jara-Pérez V (2006) Enzymatic activity assay of d-hydantoinase by isothermal titration calorimetry. Determination of the thermodynamic activation parameters for the hydrolysis of several substrates. J Biochem Biophys Methods 67(1):57–66
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL (2006) GenBank. Nucleic Acids Res 34:D16–D20
Bertani G (1951) Studies on lysogenesis.1. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62(3):293–300
Cai Y, Trodler P, Jiang S, Zhang W, Wu Y, Lu Y, Yang S, Jiang W (2009) Isolation and molecular characterization of a novel d-hydantoinase from Jannaschia sp. CCS1. FEBS J 276(13):3575–3588. doi:10.1111/j.1742-4658.2009.07077.x
Cheng RP, Gellman SH, DeGrado WF (2001) β-Peptides: from structure to function. Chem Reviews 101(10):3219–3232. doi:10.1021/cr000045i
Dakin HD, Dudley HW (1914) The resolution of inactive uramido-acids and hydantoins into active components, and their conversion into amino-acids. I. β-Phenyl-α-uramidopropionic acid, benzylhydantoin and phenylalanine. J Biol Chem 17(1):29–36
Dürr R (2007) Screening and description of novel hydantoinases from distinct environmental sources. Doctoral thesis, Universität Karlsruhe (TH), Karlsruhe, ISBN 9783866441736
Dürr R, Vielhauer O, Burton SG, Cowan DA, Punal A, Brandao PFB, Bull AT, Syldatk C (2006) Distribution of hydantoinase activity in bacterial isolates from geographically distinct environmental sources. J Mol Catal B Enzym 39(1–4):160–165
Dürr R, Neumann A, Vielhauer O, Altenbuchner J, Burton SG, Cowan DA, Syldatk C (2008) Genes responsible for hydantoin degradation of a halophilic Ochrobactrum sp. G21 and Delftia sp. 124—new insight into relation of d-hydantoinases and dihydropyrimidinases. J Mol Catal B Enzym 52–3:2–12
Fischer E, Roeder G (1901) Synthese des Uracils, Thymins und Phenyluracils. Ber Dtsch Chem Ges 34(3):3751–3763
Fleming PE, Mocek U, Floss HG (1993) Biosynthesis of taxoids. Mode of formation of the taxol side chain. J Am Chem Soc 115(2):805–807. doi:10.1021/ja00055a072
Frackenpohl J, Arvidsson PI, Schreiber JV, Seebach D (2001) The outstanding biological stability of β- and γ-peptides toward proteolytic enzymes: an in vitro investigation with fifteen peptidases. ChemBioChem 2(6):445–455
Gokhale DV, Bastawde KB, Patil SG, Kalkote UR, Joshi RR, Joshi RA, Ravindranathan T, Gaikwad BG, Jogdand VV, Nene S (1996) Chemoenzymatic synthesis of d-phenylglycine using hydantoinase of Pseudomonas desmolyticum resting cells. Enzyme Microb Technol 18(5):353–357
Kao C-H, Lo H-H, Hsu S-K, Hsu W-H (2008) A novel hydantoinase process using recombinant Escherichia coli cells with dihydropyrimidinase and l-N-carbamoylase activities as biocatalyst for the production of l-homophenylalanine. J Biotechnol 134(3–4):231
Lane D (1991) 16S/23S rRNA Sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester
Liljeblad A, Kanerva LT (2006) Biocatalysis as a profound tool in the preparation of highly enantiopure β-amino acids. Tetrahedron 62(25):5831–5854
Liu M, Sibi MP (2002) Recent advances in the stereoselective synthesis of β-amino acids. Tetrahedron 58(40):7991–8035
Louwrier A, Knowles CJ (1996) The purification and characterization of a novel d-specific carbamoylase enzyme from an Agrobacterium sp. Enzyme Microb Technol 19(8):562–571
Martínez-Gómez AI, Martínez-Rodríguez S, Clemente-Jiménez JM, Pozo-Dengra J, Rodríguez-Vico F, Las Heras-Vázquez FJ (2007) Recombinant polycistronic structure of hydantoinase process genes in Escherichia coli for the production of optically pure d-amino acids. Appl Environ Microbiol 73(5):1525–1531. doi:10.1128/aem.02365-06
Martínez-Gómez AI, Martínez-Rodríguez S, Pozo-Dengra J, Tessaro D, Servi S, Clemente-Jiménez JM, Rodríguez-Vico F, Las Heras-Vázquez FJ (2009) Potential application of N-carbamoyl-β-alanine amidohydrolase from Agrobacterium tumefaciens C58 for β-amino acid production. Appl Environ Microbiol 75(2):514–520. doi:10.1128/aem.01128-08
Martínez-Rodríguez S, Martínez-Gómez AI, Clemente-Jiménez JM, Rodríguez-Vico F, Garcia-Ruiz JM, Las Heras-Vázquez FJ, Gavira JA (2010) Structure of dihydropyrimidinase from Sinorhizobium meliloti CECT4114: new features in an amidohydrolase family member. J Struct Biol 169(2):200–208. doi:10.1016/j.jsb.2009.10.013
May O, Siemann M, Pietzsch M, Kiess M, Mattes R, Syldatk C (1998) Substrate-dependent enantioselectivity of a novel hydantoinase from Arthrobacter aurescens DSM 3745: purification and characterization as new member of cyclic amidases. J Biotechnol 61(1):1
Ogawa J, Shimizu S (1994) β-Ureidopropionase with N-carbamoyl-α-l-amino acid amidohydrolase activity from an aerobic bacterium, Pseudomonas putida IFO 12996. Eur J Biochem 223(2):625–630
Rehdorf J, Mihovilovic MD, Bornscheuer UT (2010) Exploiting the regioselectivity of Baeyer–Villiger monooxygenases for the formation of β-amino acids and β-amino alcohols. Angewandte Chemie International Edition 49(26):4506–4508. doi:10.1002/anie.201000511
Schnackerz KD, Dobritzsch D (2008) Amidohydrolases of the reductive pyrimidine catabolic pathway: purification, characterization, structure, reaction mechanisms and enzyme deficiency. Biochim Biophys Acta, Proteins Proteomics 1784(3):431–444
Scholl S, Koch A, Henning D, Kempter G, Kleinpeter E (1999) The influence of structure and lipophilicity of hydantoin derivatives on anticonvulsant activity. Struct Chem 10(5):355–366. doi:10.1023/a:1022091411018
Seebach D, Gardiner J (2008) β-Peptidic peptidomimetics. Acc Chem Res 41(10):1366–1375. doi:10.1021/ar700263g
Seebach D, Matthews JL (1997) β-Peptides: a surprise at every turn. Chem Commun (Cambridge, UK) 21:2015–2022
Siemann M, Alvarado-Marin A, Pietzsch M, Syldatk C (1999) A d-specific hydantoin amidohydrolase: properties of the metalloenzyme purified from Arthrobacter crystallopoietes. J Mol Catal B: Enzym 6(3):387
Stark GR, Smyth DG (1963) Use of cyanate for determination of NH2-terminal residues in proteins. J Biol Chem 238(1):214–226
Steer DL, Lew RA, Perlmutter P, Smith AI, Aguilar M-I (2002) β2-Amino acids: versatile peptidomimetics. Curr Med Chem 9:811
Suzuki S, Henderson PJF (2006) The hydantoin transport protein from Microbacterium liquefaciens. J Bacteriol 188(9):3329–3336. doi:10.1128/Jb.188.9.3329-3336.2006
Suzuki T, Igarashi K, Hase K, Tuzimura K (1973) Optical-rotatory dispersion and circular-dichroism of amino-acid hydantoins. Agric Biol Chem 37(2):411–416
Syldatk C, Mackowiak V, Hoke H, Gross C, Dombach G, Wagner F (1990) Cell growth and enzyme synthesis of a mutant of Arthrobacter sp. (DSM 3747) used for the production of l-amino acids from dl-5-monosubstituted hydantoins. J Biotechnol 14(3–4):345
Tasnádi G, Forró E, Fülöp F (2008) An efficient new enzymatic method for the preparation of β-aryl-β-amino acid enantiomers. Tetrahedron: Asymmetry 19(17):2072–2077
Vogels GD, van der Drift C (1976) Degradation of purines and pyrimidines by microorganisms. Bacteriological Reviews 40(2):403–468
Vuano BM, Pieroni OI, Cabaleiro MC (2000) Thermal reaction of cinnamic acid and of β-styrylphosphonic acid with urea. J Chem Res 7:318–320
Watabe K, Ishikawa T, Mukohara Y, Nakamura H (1992) Cloning and sequencing of the genes involved in the conversion of 5-substituted hydantoins to the corresponding l-amino acids from the native plasmid of Pseudomonas sp. strain NS671. J Bacteriol 174(3):962–969
Weiner B, Szymanski W, Janssen DB, Minnaard AJ, Feringa BL (2010) Recent advances in the catalytic asymmetric synthesis of β-amino acids. Chem Soc Rev 39(5):1656–1691
Werner M, Las Heras-Vazques FJ, Fritz C, Vielhauer O, Siemann-Herzberg M, Altenbuchner J, Syldatk C (2004) Cloning of d-specific hydantoin utilization genes from Arthrobacter crystallopoietes. Eng Life Sci 4(6):563–572
Wu B, Szymanski W, Wietzes P, de Wildeman S, Poelarends GJ, Feringa BL, Janssen DB (2009) Enzymatic synthesis of enantiopure α- and β-amino acids by phenylalanine aminomutase-catalysed amination of cinnamic acid derivatives. ChemBioChem 10(2):338–344. doi:10.1002/cbic.200800568
Yokozeki K, Kubota K (1987) Enzymatic production of d-amino acids from 5-substituted hydantoins: 3. Mechanism of asymmetric production of d-amino acids from the corresponding hydantoins by Pseudomonas sp. Agric Biol Chem 51(3):721–728
Acknowledgments
This work was financed by the Federal Ministry of Science and Education (BMBF), Germany. Furthermore, the authors like to thank Gerd Unkelbach from Fraunhofer Institute for Chemical Technology for cooperation in the synthesis of the above-mentioned substrates. We also thank Professor Don Cowan for providing the soil samples for the screening experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engel, U., Syldatk, C. & Rudat, J. Stereoselective hydrolysis of aryl-substituted dihydropyrimidines by hydantoinases. Appl Microbiol Biotechnol 94, 1221–1231 (2012). https://doi.org/10.1007/s00253-011-3691-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3691-7