Abstract
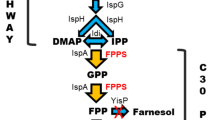
A recombinant Saccharomyces cerevisiae strain was used for the production of β-carotene. The episomal plasmid YEplac195YB/I/E was extended by a gene coding for the mevalonate kinase (mvaK1) from Staphylococcus aureus. The adh1 promoter was chosen for constitutive expression of mvaK1. The recombinant strain S. cerevisiae G175 (YEplac-CaroSA) synthesised β-carotene by expressing the carotenogenic genes of Xanthophyllomyces dendrorhous together with the mvaK1 gene. Cells of this strain were investigated for their carotenoid contents in YNB and YPD media. A corresponding mvaK1 transcript in the recombinant yeast host was verified. Growth experiments of a specific erg12 deletion mutant showed that the mevalonate kinase (MvaK1) was able to complement the function of the deleted native mevalonate kinase (Erg12) from S. cerevisiae in the MVA pathway under control of the constitutive adh1 promoter. Cells of S. cerevisiae G175 (YEplac-CaroSA) exhibited high plasmid stability under either selective or non-selective cultivation conditions. Time course experiments demonstrated high plasmid stability even over extended cultivation periods. Carotenoid production was therefore also stable in larger culture volumes. Due to the stability of the plasmid, cultivation of the cells in complex YPD medium was possible, and 14.3 mg β-carotene per litre and a cell density of 9 g cell dry matter (CDM) per litre were achieved. The highest amount of 3,897 μg β-carotene per gramme CDM at a cell density of 1 g CDM per litre was measured after cultivation of the cells in YNB medium with glucose as sole carbon source.





Similar content being viewed by others
References
Armstrong GA, Alberti M, Leach F, Hearst JE (1989) Nucleotide sequence, organization and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet 216:254–268
Armstrong GA, Alberti M, Hearst JE (1990) Conserved enzymes mediate the early reactions of carotenoid biosynthesis in nonphotosynthetic and photosynthetic prokaryotes. Proc Natl Acad Sci U S A 87:9975–9979
Britton G, Liaaen-Jensen S, Pfander H (1995) Carotenoids volume 1a: isolation and analysis. Birkhäuser, Basel, Switzerland
Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids handbook, 1st edn. Birkhäuser, Basel, Switzerland
Cherry JM, Ball C, Weng S, Juvik G, Schmidt R, Adler C, Dunn B, Dwight S, Riles L, Mortimer RK, Botstein D (1997) Genetic and physical maps of Saccharomyces cerevisiae. Nature 387:67–73
Frengova GI, Beshkova DM (2009) Carotenoids from Rhodotorula and Phaffia: yeasts of biotechnological importance. J Ind Microbiol Biotechnol 36:163–180
Futcher AB, Cox BS (1984) Copy number and the stability of 2-micron circle-based artificial plasmids of Saccharomyces cerevisiae. J Bacteriol 157:283–290
Gietz RD, Woods RA (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Meth Enzymol 350:87–96
Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, Feldmann H, Galibert F, Hoheisel JD, Jacq C, Johnston M, Louis EJ, Mewes HW, Murakami Y, Philippsen P, Tettelin H, Oliver SG (1996) Life with 6000 genes. Science 274(546):563–567
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425–430
Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH (2002) A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res 30:e23
Huang B, Guo J, Yi B, Yu X, Sun L, Chen W (2008) Heterologous production of secondary metabolites as pharmaceuticals in Saccharomyces cerevisiae. Biotechnol Lett 30:1121–1137
Kirby J, Keasling JD (2009) Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu Rev Plant Biol 60:335–355
Kroll J, Steinle A, Reichelt R, Ewering C, Steinbüchel A (2009) Establishment of a novel anabolism-based addiction system with an artificially introduced mevalonate pathway: complete stabilization of plasmids as universal application in white biotechnology. Metab Eng 11:168–177
Kroll J, Klinter S, Schneider C, Voß I, Steinbüchel A (2010) Plasmid addiction systems: perspectives and applications in biotechnology. Microb Biotechnol 3:634–657
Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Biol 50:47–65
Liu GN, Zhu YH, Jiang JG (2009) The metabolomics of carotenoids in engineered cell factory. Appl Microbiol Biotechnol 83:989–999
Lynen F (1967) Biosynthetic pathways from acetate to natural products. Pure Appl Chem 14:137–167
Mewes HW, Albermann K, Bahr M, Frishman D, Gleissner A, Hani J, Heumann K, Kleine K, Maierl A, Oliver SG, Pfeiffer F, Zollner A (1997) Overview of the yeast genome. Nature 387:7–65
Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K (1990) Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol 172:6704–6712
Misawa N, Yamano S, Ikenaga H (1991) Production of beta-carotene in Zymomonas mobilis and Agrobacterium tumefaciens by introduction of the biosynthesis genes from Erwinia uredovora. Appl Environ Microbiol 57:1847–1849
Miziorko HM (2011) Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys 505:131–143
Partow S, Siewers V, Bjorn S, Nielsen J, Maury J (2010) Characterization of different promoters for designing a new expression vector in Saccharomyces cerevisiae. Yeast 27:955–964
Primrose SB, Ehrlich SD (1981) Isolation of plasmid deletion mutants and study of their instability. Plasmid 6:193–201
Rohmer M, Knani M, Simonin P, Sutter B, Sahm H (1993) Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J 295(Pt 2):517–524
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual 2nd edition. Cold Spring Harbor Laboratory Press, NY
Sandager L, Gustavsson MH, Stahl U, Dahlqvist A, Wiberg E, Banas A, Lenman M, Ronne H, Stymne S (2002) Storage lipid synthesis is non-essential in yeast. J Biol Chem 277:6478–6482
Scholz M, Lipinski M, Leupold M, Luftmann H, Harig L, Ofir R, Fischer R, Prüfer D, Müller KJ (2009) Methyl jasmonate induced accumulation of kalopanaxsaponin I in Nigella sativa. Phytochemistry 70:517–522
Ugolini S, Tosato V, Bruschi CV (2002) Selective fitness of four episomal shuttle-vectors carrying HIS3, LEU2, TRP1, and URA3 selectable markers in Saccharomyces cerevisiae. Plasmid 47:94–107
Ukibe K, Hashida K, Yoshida N, Takagi H (2009) Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance. Appl Environ Microbiol 75:7205–7211
Verwaal R, Wang J, Meijnen JP, Visser H, Sandmann G, van den Berg JA, van Ooyen AJ (2007) High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl Environ Microbiol 73:4342–4350
Voynova NE, Rios SE, Miziorko HM (2004) Staphylococcus aureus mevalonate kinase: isolation and characterization of an enzyme of the isoprenoid biosynthetic pathway. J Bacteriol 186:61–67
Vu VH, Kim K (2009) High-cell-density fed-batch culture of Saccharomyces cerevisiae KV-25 using molasses and corn steep liquor. J Microbiol Biotechnol 19:1603–1611
Walker GM (1998) Yeast physiology and biotechnology. John Wiley & Sons, New York, USA
Yamano S, Ishii T, Nakagawa M, Ikenaga H, Misawa N (1994) Metabolic engineering for production of beta-carotene and lycopene in Saccharomyces cerevisiae. Biosci Biotechnol Biochem 58:1112–1114
Yoon S, Park H, Kim J, Lee S, Choi M, Kim J, Oh D, Keasling JD, Kim S (2007) Increased β-carotene production in recombinant Escherichia coli harboring an engineered isoprenoid precursor pathway with mevalonate addition. Biotechnol Prog 23:599–605
Yoon S, Lee S, Das A, Ryu H, Jang H, Kim J, Oh D, Keasling JD, Kim S (2009) Combinatorial expression of bacterial whole mevalonate pathway for the production of β-carotene in E. coli. J Biotechnol 140:218–226
Zhang Z, Moo-Young M, Chisti Y (1996) Plasmid stability in recombinant Saccharomyces cerevisiae. Biotechnol Adv 14:401–435
Acknowledgements
We thank Rene Verwaal (DSM Biotechnology Centre, Delft, The Netherlands) for the generous provision of the yeast plasmids for β-carotene production. We thank Roland Klassen and Friedhelm Meinhardt (Institut für Molekulare Mikrobiologie und Biotechnologie, Münster, Germany) for providing vector pUG73 and Kai Müller (Fraunhofer Institut für Molekularbiologie und Angewandte Ökologie, Aachen, Germany) for providing vector PADNsβAS1. We also thank M. Gustavsson, E. Wiberg, P. Stolt and S. Stymne (Scandinavian Biotechnology Research, Alnarp, Sweden) for providing S. cerevisiae G175.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lange, N., Steinbüchel, A. β-Carotene production by Saccharomyces cerevisiae with regard to plasmid stability and culture media. Appl Microbiol Biotechnol 91, 1611–1622 (2011). https://doi.org/10.1007/s00253-011-3315-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3315-2