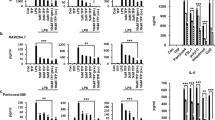
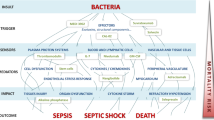
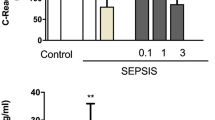
Abstract
Sepsis (blood poisoning) is a severe infectious disease with high mortality, and no effective therapy is actually known. In the case of Gram-negative bacteria, endotoxins (lipopolysaccharides) are known to be responsible for the strong inflammation reaction leading to the systemic infection. Peptides based on endotoxin-binding domains of human or animal proteins represent a promising approach in sepsis research. Although so far no medicament is available, the progress in recent years might lead to a breakthrough in this field. In this review, recent investigations are summarised, which may lead to an understanding of the mechanisms of action of peptides to suppress the inflammation reaction in vitro and in vivo (animal models) and thus may allow the development of effective anti-septic drugs.



Similar content being viewed by others
References
Agerberth B, Grunewald J, Castanos-Velez E, Olsson B, Jornvall H, Wigzell H, Eklund A, Gudmundsson GH (1999) Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med 160:283–290
Andersson M, Gunne H, Agerberth B, Boman A, Bergman T, Sillard R, Jornvall H, Mutt V, Olsson B, Wigzell H (1995) NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J 14:1615–1625
Andersson M, Gunne H, Agerberth B, Boman A, Bergman T, Olsson B, Dagerlind A, Wigzell H, Boman HG, Gudmundsson GH (1996) NK-lysin, structure and function of a novel effector molecule of porcine T and NK cells. Vet Immunol Immunopathol 54:123–126
Andersson M, Girard R, Cazenave P (1999) Interaction of NK lysin, a peptide produced by cytolytic lymphocytes, with endotoxin. Infect Immun 67:201–205
Andrä J, Garidel P, Majerle A, Jerala R, Ridge R, Paus E, Novitsky T, Koch MHJ, Brandenburg K (2004a) Biophysical characterization of the interaction of Limulus polyphemus endotoxin neutralizing protein with lipopolysaccharide. Eur J Biochem 271:2037–2046
Andrä J, Koch MHJ, Bartels R, Brandenburg K (2004b) Biophysical characterization of endotoxin inactivation by NK-2, an antimicrobial peptide derived from mammalian NK-lysin. Antimicrob Agents Chemother 48:1593–1599
Andrä J, Lamata M, Martinez de Tejada G, Bartels R, Koch MHJ, Brandenburg K (2004c) Cyclic antimicrobial peptides based on Limulus anti-lipopolysaccharide factor for neutralization of lipopolysaccharide. Biochem Pharmacol 68:1297–1307
Andrä J, Lohner K, Blondelle SE, Jerala R, Moriyon I, Koch MHJ, Garidel P, Brandenburg K (2005) Enhancement of endotoxin neutralization by coupling of a C12-alkyl chain to a lactoferricin-derived peptide. Biochem J 385:135–143
Andrä J, Gutsmann T, Garidel P, Brandenburg K (2006) Mechanisms of endotoxin neutralization by synthetic cationic compounds. J Endotoxin Res 12:261–277
Andrä J, Howe J, Garidel P, Rössle M, Richter W, Leiva-Leon J, Moriyon I, Bartels R, Gutsmann T, Brandenburg K (2007a) Mechanism of interaction of optimized Limulus-derived cyclic peptides with endotoxins: thermodynamic, biophysical and microbiological analysis. Biochem J 406:297–307
Andrä J, Monreal D, Martinez de Tejada G, Olak C, Brezesinski G, Gomez SS, Goldmann T, Bartels R, Brandenburg K, Moriyon I (2007b) Rationale for the design of shortened derivatives of the NK-lysin-derived antimicrobial peptide NK-2 with improved activity against Gram-negative pathogens. J Biol Chem 282:14719–14728
Appelmelk BJ, An Y-Q, Geerts M, Thijs BG, De Boer HA, MacLaren DM, De Graaff J, Nuijens JH (1994) Lactoferrin is a lipid A-binding protein. Infect Immun 62:2628–2632
Baumgartner JD, Heumann D, Gerain J, Weinbreck P, Grau GE, Glauser MP (1990) Association between protective efficacy of anti-lipopolysaccharide (LPS) antibodies and suppression of LPS-induced tumor necrosis factor alfa and interleukin 6. J Exp Med 171:889–896
Bowdish DM, Hancock RE (2005) Anti-endotoxin properties of cationic host defence peptides and proteins. J Endotoxin Res 11:230–236
Brandenburg K, Jürgens G, Müller M, Fukuoka S, Koch MHJ (2001) Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol Chem 382:1215–1225
Brandenburg K, David A, Howe J, Koch MHJ, Andrä J, Garidel P (2005) Temperature dependence of the binding of endotoxins to the polycationic peptides polymyxin B and its nonapeptide. Biophys J 88:1845–1858
Brandenburg K, Garidel P, Fukuoka S, Howe J, Koch MHJ, Gutsmann T, Andrä J (2010) Molecular basis for endotoxin neutralization by amphipathic peptides derived from the alpha-helical cationic core-region of NK-lysin. Biophys Chem 150:80–87
Chen X, Howe J, Andrä J, Rössle M, Richter W, da Silva AP, Krensky AM, Clayberger C, Brandenburg K (2007) Biophysical analysis of the interaction of granulysin-derived peptides with enterobacterial endotoxins. Biochim Biophys Acta 1768:2421–2431
Ciornei CD, Sigurdardottir T, Schmidtchen A, Bodelsson M (2005) Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob Agents Chemother 49:2845–2850
Cowland JB, Johnsen AH, Borregaard N (1995) hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett 368:173–176
Cross AS, Opal SM (1995) Endotoxin's role in Gram-negative bacterial infection. Curr Opin Infect Dis 8:156–163
Cruz DN, Bellomo R, Ronco C (2007) Clinical effects of polymyxin B-immobilized fiber column in septic patients. Contrib Nephrol 156:444–451
Dankesreiter S, Hoess A, Schneider-Mergener J, Wagner H, Mietke T (2000) Synthetic endotoxin-binding peptides block endotoxin-triggered TNF-α production by macrophages in vitro and in vivo and prevent endotoxin-mediated toxic shock. J Immunol 164:4804–4811
Davis EG, Sang Y, Rush B, Zhang G, Blecha F (2005) Molecular cloning and characterization of equine NK-lysin. Vet Immunol Immunopathol 105:163–169
de Haas CJ, Haas PJ, van Kessel KP, van Strijp JA (1998) Affinities of different proteins and peptides for lipopolysaccharide as determined by biosensor technology. Biochem Biophys Res Commun 252:492–496
Deng A, Chen S, Li Q, Lyu SC, Clayberger C, Krensky AM (2005) Granulysin, a cytolytic molecule, is also a chemoattractant and proinflammatory activator. J Immunol 174:5243–5248
Dhainaut JF, Yan SB, Joyce DE, Pettila V, Basson B, Brandt T, Sundin DP, Levis ML (2004) Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost 2:1924–1833
Elass-Rochard E, Legrand D, Salmon V, Roseanu A, Trif M, Tobias PS, Mazurier J, Spik G (1998) Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect Immun 66:486–491
Endsley JJ, Furrer JL, Endsley MA, McIntosh MA, Maue AC, Waters WR, Lee DR, Estes DM (2004) Characterization of bovine homologues of granulysin and NK-lysin. J Immunol 173:2607–2614
Falagas ME, Kasiakou SK (2006) Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 10:R27
Frohm M, Gunne H, Bergman AC, Agerberth B, Bergman T, Boman A, Liden S, Jornvall H, Boman HG (1996) Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem 237:86–92
Garidel P, Brandenburg K (2009) Current understanding of polymyxin B applications in bacteraemia/sepsis therapy prevention: clinical, pharmaceutical, structural and mechanistic aspects. Antiinfect Agents Medic Chem 8:367–385
Gutsmann T, Howe J, Zähringer U, Garidel P, Schromm AB, Koch MHJ, Fujimoto Y, Fukase K, Moriyon I, Martinez de Tejada G, Brandenburg K (2010a) Structural prerequisites for endotoxic activity in the Limulus test as compared to cytokine production in mononuclear cells. Innate Immun 16:39–47
Gutsmann T, Razquin-Olazaran I, Kowalski I, Kaconis Y, Howe J, Bartels R, Hornef M, Schürholz T, Rössle M, Sanchez-Gomez S, Moriyon I, Martinez de Tejada G, Brandenburg K (2010b) New antiseptic peptides to protect against endotoxin-mediated shock. Antimicrob Agents Chemother 54:3817–3824
Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557
Hashimoto M, Furuyashiki M, Kaseya R, Fukada Y, Akimaru M, Aoyama K, Okuno T, Tamura T, Kirikae T, Kirikae F, Eiraku N, Morioka H, Fujimoto Y, Fukase K, Takashige K, Moriya Y, Kusumoto S, Suda Y (2007) Evidence of immunostimulating lipoprotein existing in the natural lipoteichoic acid fraction. Infect Immun 75:1926–1932
Haversen L, Kondori N, Baltzer L, Hanson LA, Dolphin GT, Duner K, Mattsby-Baltzer I (2010) Structure-microbicidal activity relationship of synthetic fragments derived from the antibacterial alpha-helix of human lactoferrin. Antimicrob Agents Chemother 54:418–425
Hirata M, Zhong J, Wright SC, Larrick JW (1995) Structure and functions of endotoxin-binding peptides derived from CAP18. Prog Clin Biol Res 392:317–326
Hoess A, Watson S, Siber GR, Liddington R (1993) Crystal structure of an endotoxin-neutralizing protein from the horseshoe crab, Limulus anti-LPS factor, at 1.5 A resolution. EMBO J 12:3351–3356
Hornef MW, Wick MJ, Rhen M, Normark S (2002) Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol 3:1033–1040
Howe J, Andrä J, Conde R, Iriarte M, Garidel P, Koch MHJ, Gutsmann T, Moriyon I, Brandenburg K (2007) Thermodynamic analysis of the lipopolysaccharide-dependent resistance of gram-negative bacteria against polymyxin B. Biophys J 92:2796–2805
Japelj B, Pristovsek P, Majerle A, Jerala R (2005) Structural origin of endotoxin neutralization and antimicrobial activity of a lactoferrin-based peptide. J Biol Chem 280:16955–16961
Kowalski I, Kaconis Y, Andrä J, Razquin-Olazaran I, Gutsmann T, Martinez de Tejada G, Brandenburg K (2010) Physicochemical and biological characterization of anti-endotoxin peptides and their Influence on lipid properties. Protein Pept Lett 17:1328–1333
Larrick JW, Morgan JG, Palings I, Hirata M, Yen MH (1991) Complementary DNA sequence of rabbit CAP18–a unique lipopolysaccharide binding protein. Biochem Biophys Res Commun 179:170–175
Lefrant JY, Muller L, Raillard A, Jung B, Beaudroit L, Favier L, Masson B, Dingemans G, Thevenot F, Selcer D, Jonquet O, Capdevila X, Fabbro-Peray P, Jaber S (2010) Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Rèanim 29:621–628
Lehrer RI, Ganz T (2002) Defensins of vertebrate animals. Curr Opin Immunol 14:96–102
Leippe M (1995) Ancient weapons: NK-lysin is a mammalian homolog to pore-forming peptides of a protozoan parasite. Cell 83:17–18
Levin J (1987) The Limulus amebocyte lysate test: perspectives and problems. Prog Clin Biol Res 231:1–23
Liepinsh E, Andersson M, Ruysschaert JM, Otting G (1997) Saposin fold revealed by the NMR structure of NK-lysin. Nat Struct Biol 4:793–795
Malm J, Sorensen O, Persson T, Frohm-Nilsson M, Johansson B, Bjartell A, Lilja H, Stahle-Backdahl M, Borregaard N, Egesten A (2000) The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun 68:4297–4302
Michalopoulos A, Falagas ME (2008) Colistin and polymyxin B in critical care. Crit Care Clin 24:377–391
Morrison DC (1998) Antibiotic-mediated release of endotoxin and the pathogenesis of Gram-negative sepsis. Prog Clin Biol Res 397:199–207
Motzkus D, Schulz-Maronde S, Heitland A, Schulz A, Forssmann WG, Jubner M, Maronde E (2006) The novel beta-defensin DEFB123 prevents lipopolysaccharide-mediated effects in vitro and in vivo. FASEB J 20:1701–1702
Ogata M, Fletcher MF, Kloczewiak M, Loiselle PM, Zanzot EM, Vermeulen MW, Warren HS (1997) Effect of anticoagulants on binding and neutralization of lipopolysaccharide by the peptide immunoglobulin conjugate CAP18106–138-immunoglobulin G in whole blood. Infect Immun 65:2160–2167
Opal SM, Cohen J (1999) Clinical Gram-positive sepsis: does it fundamentally differ from Gram-negative bacterial sepsis? Crit Care Med 27:1608–1616
Pan CY, Chao TT, Chen JC, Chen JY, Liu WC, Lin CH, Kuo CM (2007) Shrimp (Penaeus monodon) anti-lipopolysaccharide factor reduces the lethality of Pseudomonas aeruginosa sepsis in mice. Int Immunopharmacol 7:687–700
Pena SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM (1997) Processing, subcellular localization, and function of 519 (Granulysin), a human late T cell sctivation molecule with homology to small, lytic, granule proteins. J Immunol 158: 2680–2688
Pini A, Falciani C, Mantengoli E, Bindi S, Brunetti J, Iozzi S, Maria RG, Bracci L (2009) A novel tetrabranched antimicrobial peptide that neutralizes bacterial lipopolysaccharide and prevents septic shock in vivo. FASEB J 24:1015–1022
Reinhart K, Karzai W (2001) Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med 7:S121–S125
Ren JD, Gu JS, Gao HF, Xia PY, Xiao GX (2008) A synthetic cyclic peptide derived from Limulus anti-lipopolysaccharide factor neutralizes endotoxin in vitro and in vivo. Int Immunopharmacol 8:775–781
Ried C, Wahl C, Miethke T, Wellnhofer G, Landgraf C, Schneider-Mergener J, Hoess A (1996) High affinity endotoxin-binding and neutralizing peptides based on the crystal structure of recombinant Limulus anti-lipopolysaccharide factor. J Biol Chem 271:28120–28127
Scott MG, Vreugdenhil ACE, Buurman WA, Hancock REW, Gold M (2000) Cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol 164:549–553
Sorensen O, Cowland JB, Askaa J, Borregaard N (1997) An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Meth 206:53–59
Tidswell M, Tillis W, LaRosa SP, Lynn M, Wittek AE, Kao R, Wheller J, Gogate J, Opal SM, Eritoran Sepsis Study Group (2010) Phase 2 trial od eritoran tetrasodium (E5564), a Toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med 38:72–83
Travis SM, Anderson NN, Forsyth WR, Espiritu C, Conway BD, Greenberg EP, McCray PB, Lehrer RI, Welsh MJ, Tack BF (2000) Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun 68:2748–2755
Wang Q, Wang Y, Xu P, Liu Z (2006) NK-lysin of channel catfish: gene triplication, sequence variation, and expression analysis. Mol Immunol 43:1676–1686
Wieczorek M, Jenssen H, Kindrachuk J, Scott WR, Elliott M, Hilpert K, Cheng JT, Hancock RE, Straus SK (2010) Structural studies of a peptide with immune modulating and direct antimicrobial activity. Chem Biol 17:970–980
Yang SH, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, Takada H (2001) Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun 69:2045–2053
Zanetti M, Gennaro R, Romeo D (1995) Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett 374:1–5
Acknowledgements
The authors are indebted the German ministry BMBF for financial support (K.B. 01GU0824). J.A. acknowledges the financial support from the German Science Foundation (DFG) grant AN301/5-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brandenburg, K., Andrä, J., Garidel, P. et al. Peptide-based treatment of sepsis. Appl Microbiol Biotechnol 90, 799–808 (2011). https://doi.org/10.1007/s00253-011-3185-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3185-7