Abstract
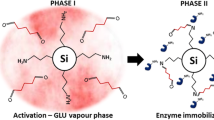
The covalent immobilization of laccase on an inorganic ceramic support was investigated. The intention was to find a system of enzyme and reactor for a universal immobilization procedure. Laccase from Trametes versicolor as model enzyme was chosen. The special honeycomb structure of the monolith can be applied for intensive mixing of the reaction compounds. An appropriate reactor with ceramic material was constructed allowing different setup for enzyme immobilization and its application. To test the success of the immobilization, 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) was used. The immobilized laccase was found to be stable over a time period of over 3 months. As an example for possible application for treatment of wastewater containing dyes, the conversion of nuclear fast red as model substrate was tested.







Similar content being viewed by others
References
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30:215–242
Bautista LF, Morales G, Sanz R (2010) Immobilization strategies for laccase from Trametes versicolor on mesostructured silica materials and the application to the degradation of naphthalene. Bioresour Technol 101:8541–8548
Boger T, Roy S, Heibel A, Borchers O (2003) A monolith loop reactor as an attractive alternative to slurry reactors. Catal Today 79:441–451
Champagne PP, Ramsay JA (2010) Dye decolorization and detoxification by laccase immobilized on porous glass beads. Bioresour Technol 101:2230–2235
Cho N, Cho H, Shin S, Choi Y, Leonowicz A, Ohga S (2008) Production of fungal laccase and its immobilization and stability. J Fac Agric Kyushu Univ 53:13–18
de Lathouder KM, Flo TM, Kapteijn F, Moulijn JA (2005) A novel structured bioreactor: Development of a monolithic stirrer reactor with immobilized lipase. Catal Today 105:443–447
de Lathouder KM, Smeltink MW, Straathof AJJ, Paasman MA, van de Sandt E, Kapteijn F, Moulijn JA (2008) Hydrogel coated monoliths for enzymatic hydrolysis of penicillin G. J Ind Microbiol Biotechnol 35:815–824
Dodor DE, Hwang HM, Ekunwe SIN (2004) Oxidation of anthracene and benzo[a]pyrene by immobilized laccase from Trametes versicolor. Enzyme Microb Technol 35:210–217
Duran N, Rosa MA, D’Annibale A, Gianfreda L (2002) Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzyme Microb Technol 31:907–931
Ghommidh J, Navarro J, Messing R (1982) A study of acetic acid production by immobilized Acetobacter cells: product inhibition effects. Biotechnol Bioeng 24:1991–1999
Hartmeier W (1986) Immobilisierte Biokatalysatoren. Springer, Berlin Heidelberg New York Tokio
He F, Zhuo RX, Liu LJ, Xu MY (2000) Immobilization of acylase on porous silica beads: preparation and thermal activation studies. React Funct Polym 45:29–33
Horn A, Kumar S, Liese A, Kragl U (2008) In: Ertl G, Knözinger H, Schüth F, Weitkamp J (eds) Handbook of heterogeneous catalysis. Wiley-VCH, Weinheim, pp 3831–3865
Kawakami K, Adachi K, Minemura N, Kusunoki K (1989) Characteristics of a gas–liquid–solid, three-phase, honeycomb-monolith bioreactor. Oxydation of glucose by immobilized glucose oxidase. Int Chem Eng 29:320–327
Kragl U (2003) Biocatalysis. In: Baerns M (ed) Basic principles in applied catalysis. Springer, Berlin, pp 443–452
Kragl U, Liese A (1999) Biotransformations, engineering aspects. In: Flickinger MC, Drew SW (eds) Encyclopedia of bioprocess technology: fermentation, biocatalysis and bioseparation. Wiley, New York, pp 454–464
Kunamneni A, Ghazi I, Camarero S, Ballesteros A, Plou FJ, Alcalde M (2008) Decolorization of synthetic dyes by laccase immobilized on epoxy-activated carriers. Process Biochem 43:169–178
Leonowicz A, Sarkar JM, Bollag JM (1988) Improvement in stability of an immobilized fungal laccase. Appl Microbiol Biotechnol 29:129–135
Osma JF, Toca-Herrera JL, Rodriguez-Couto S (2010) Biodegradation of a simulated textile effluent by immobilised-coated laccase in laboratory-scale reactors. Appl Catal A 373:147–153
Pilz R, Hammer E, Schauer F, Kragl U (2003) Laccased-catalysed synthesis of coupling products of phenolic substrates in different reactors. Appl Microbiol Biotechnol 60:708–712
Roy-Arcand L, Archibald FS (1991) Direct dechlorination of chlorophenolic compounds by laccases from Trametes (Coriolus) versicolor. Enzyme Microb Technol 13:194–203
Sakai T (2004) Low-temperature method for a dramatic improvement in enantioselectivity in lipase-catalyzed reactions. Tetrahydron: Asymmetry 15:2749–2756
Schliephake K, Mainwaring DE, Lonergan GT, Jones IK, Baker WL (2000) Transformation and degradation of the disazo dye Chicago Sky Blue by a purified laccase from Pycnoporus cinnabarinus. Enzyme Microb Technol 27:100–107
Sheldon RA (2007) Enzyme immobilization: the quest for optimum performance. Adv Synth Catal 349:1289–1307
Shirashi F, Kawakami K, Kono S, Tamura A, Tsurata S, Kusonoki K (1989) Characterization of production of free gluconic acid by Gluconbacter suboxydans adsorbed on ceramic honeycomb monolith. Biotechnol Bioeng 33:1413–1418
Weetall HH, Mason RD (1973) Studies on immobilized papain. Biotchnol Bioeng 15:455–466
Yinghui D, Qiuling W, Shiyu F (2002) Laccase stabilization by covalent binding immobilization on activated polyvinyl alcohol carrier. Lett Appl Microbiol 35:451–456
Acknowledgements
This work is financially supported by Deutsche Forschungsgemeinschaft (KR 2491/2-1). We thank Peter Kumm for his excellent support by building the reactor and technical equipment. We are grateful to Corning GmbH for providing the monolith samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plagemann, R., Jonas, L. & Kragl, U. Ceramic honeycomb as support for covalent immobilization of laccase from Trametes versicolor and transformation of nuclear fast red. Appl Microbiol Biotechnol 90, 313–320 (2011). https://doi.org/10.1007/s00253-010-3038-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-3038-9