Abstract
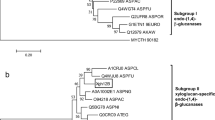
We have cloned three putative endoglucanase cDNAs, designated MoCel12A, MoCel12B, and MoCel12C, from Magnaporthe oryzae. The deduced peptide sequences of both MoCel12A and MoCel12B contain secretion signal peptides and a catalytic core domain that classify them into GH subfamily 12-1. In contrast, the deduced peptide sequence of MoCel12C consists of a signal peptide, a catalytic core domain, and a fungal-type carbohydrate binding module belonging to GH subfamily 12-2. Although most GH family 12 endoglucanases hydrolyze β-1,4-glucans such as carboxymethylcellulose or phosphoric acid-swollen cellulose, MoCel12A that was prepared by overexpression in M. oryzae and Brevibacillus choshinensis hydrolyzed specifically 1,3–1,4-β-glucans, such as barley β-glucan and lichenan. The specific activity of MoCel12A overexpressed in M. oryzae was about 20 times higher than that prepared from B. choshinensis. Furthermore, MoCel12B prepared by overexpression in B. choshinensis also revealed preferential hydrolysis of endo-1,3–1,4-β-glucans with limited hydrolysis on carboxymethylcellulose. In comparison with MoCel12A, the activity of MoCel12B was more stable under alkaline conditions. Levels of mRNA encoding MoCel12A were constitutively high during infection and spore formation. The overexpression and disruption of the MoCel12A gene did not affect germination, appressorium formation, or invasion rate; however, M. oryzae overexpressing MoCel12A produced larger numbers of spores than the wild type or a mutant in which the MoCel12A gene was disrupted. These results suggest that MoCel12A functions in part to hydrolyze 1,3–1,4-β-glucan during infection and spore formation.






Similar content being viewed by others
References
Annis SL, Goodwin PH (1997) Recent advances in the molecular genetics of plant cell wall-degrading enzymes produced by plant pathogenic fungi. Eur J Plant Pathol 103:1–14
Becherman JL, Ebbole DJ (1996) MPG1, a gene encoding a fungal hydrophobin of Magnaporthe grisea, is involved in surface recognition. Mol Plant-Microb Interact 9:450–456
Béguin P, Aubert JP (1994) The biological degradation of cellulose. FEMS Microbiol Rev 13:25–58
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383
Byeong-Cheol S, Kim K, Yoon J, Sim S, Lee K, Kim Y, Kim Y, Cha C (2008) Functional analysis of a gene encoding endoglucanase that belongs to glycosyl hydrolase family 12 from the brown-rot basidiomycete Fomitopsis palustris. J Microbiol Biotechnol 18:404–409
Campbell P, Braam J (1998) Co- and/or post-translatonal modifications are critical for TCH4 XET activity. Plant J 15:553–561
Carpita NC (1996) Structure and biogenesis of the cell walls of grasses. Annu Rev Plant Physiol Plant Mol Biol 47:445–476
Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407:321–326
Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu JR, Pan H, Read ND, Lee YH, Carbone I, Brown D, Oh YY, Donofrio N, Jeong JS, Soanes DM, Djonovic S, Kolomiets E, Rehmeyer C, Li W, Harding M, Kim S, Lebrun MH, Böhnert H, Coughlan S, Butler J, Calvo S, Ma LJ, Nicol R, Purcell S, Nusbaum C, Galagan JE, Birren BW (2005) The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434:980–986
Foster AJ, Jenkinson JM, Talbot NJ (2003) Trehalose synthesis and metabolism are required at different stages of plant infection by Magnaporthe grisea. EMBO J 22:225–235
Goedegebuur F, Fowler T, Phillips J, van der Kley P, Solingen P, Dankmeyer L, Power SD (2002) Cloning and relational analysis of 15 novel fungal endoglucanases from family 12 glycosyl hydrolase. Curr Genet 41:89–98
Görlach JM, van der Knaap E, Walton JD (1998) Cloning and targeted disruption of MLG1, a gene encoding two of three extracellular mixed-linked glucanases of Cochliobolus carbonum. Appl Environ Microbiol 64:385–391
Hahn M, Olsen O, Politz O, Borriss R, Heinemann U (1995) Crystal structure and site-directed mutagenesis of Bacillus macerans endo-1, 3–1, 4-β-glucanase. J Biol Chem 270:3081–3088
Harwood CR, Cranenburgh R (2008) Bacillus protein secretion: an unfolding story. Trends Microbiol 16:73–79
Henrissat B, Bairoch A (1996) Updating the sequence-based classification of glycosyl hydrolases. Biochem J 316:695–696
Henrissat B, Davies G (1997) Structure and sequence-based classification of glycoside hydrolases. Curr Opin Biol 7:637–644
Kim H, Ahn J, Görlach JM, Caprari C, Scott-Craig JS, Walton JD (2001) Mutational analysis of β-glucanase genes from the plant-pathogenic fungus Cochliobolus carbonum. Mol Plant-Microbe Interact 14:1436–1443
Maurer KH (1997) Development of new cellulases. In: van Ee JH, Misset O, Baas EJ (eds) Enzymes in detergency. Marcel Dekker, New York, pp 175–202
Miller M (1972) A new reaction for colorimetric determination of carbohydrates. Anal Biochem 47:273–279
Nakazawa H, Okada K, Onodera T, Ogasawara W, Okada H, Morikawa Y (2009) Directed evolution of endoglucanase III (Cel12A) from Trichoderma reesei. Appl Microbiol Biotechnol 83:649–657
Okada H, Tada K, Sekiya T, Yokoyama K, Takahashi A, Tohda H, Kumagai H, Morikawa Y (1998) Molecular characterization and heterologous expression of the gene encoding a low-molecular-mass endoglucanase from Trichoderma reesei QM9414. Appl Environ Microbiol 64:555–563
Okada H, Mori K, Tada K, Nogawa M, Morikawa Y (2000) Identification of active site carboxylic residues in Trichoderma reesei endoglucanase Cel12A by site-directed mutagenesis. J Mol Catal B, Enzym 10:249–255
Ooi T, Shinmyo A, Okada H, Hara S, Ikenaka T, Murao S, Arai M (1990) Cloning and sequence analysis of a cDNA for cellulase (FI-CMCase) from Aspergillus aculeatus. Curr Genet 18:217–222
Pauly M, Andersen LN, Kauppinen S, Kofod LV, York WS, Albersheim P, Darvill A (1999) A xyloglucan-specific endo-β-1, 4-glucanase from Aspergillus aculeatus: expression cloning in yeast, purification and characterization of the recombinant enzyme. Glycobiol 9:93–100
Purugganan MM, Braam J, Fry SC (1997) The Arabidopsis TCH4 xyloglucan endotransglycosylae. Plant Physiol 115:181–190
Sandgren M, Shaw A, Ropp TH, Wu S, Bott R, Cameron AD, Ståhlberg J, Mitchinson C, Jones TA (2001) The X-ray crystal structure of the Trichoderma reesei family 12 endoglucanase 3, Cel12A, at 1.9 Å resolution. J Mol Biol 308:295–310
Sandgren M, Gualfetti PJ, Paech C, Paech S, Shaw A, Gross LS, Saldajeno M, Berglund GI, Jones TA, Mitchinson C (2003) The Humicola grisea Cel12A enzyme structure at 1.2 Å resolution and the impact of its free cysteine residues on thermal stability. Protein Sci 12:2782–2793
Schülein M (1997) Enzymatic properties of cellulases from Humicola insolens. J Biotechnol 57:71–81
Scott-Craig JS, Panaccione DG, Cervone F, Walton JD (1990) Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell 2:1191–1200
Sherwood RT, Vance CP (1990) Resistance to fungal penetration in Gramineae. Phytopathology 70:273–279
Sulzenbacher G, Sharech F, Morosoli R, Dupont C, Davies GJ (1997) The Streptomyces lividans family 12 endoglucanase: Construction of the catalytic core, expression, and X-ray structure at 1.75 Å resolution. Biochemistry 36:16032–16039
Takano Y, Komeda K, Kojima K, Okuno T (2001) Proper regulation of cyclic AMP-dependent protein kinase is required for growth, conidiation, and appressorium function in the anthracnose fungus Colletotrichum lagenarium. Mol Plant Microb Interact 14:1149–1157
Takeda T, Furuta Y, Awano T, Mizuno K, Mitsuishi Y, Hayashi T (2002) Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proc Natl Acad Sci USA 99:9055–9060
Tamoi M, Kurotaki H, Fukamizo T (2007) β-1, 4-glucanase-like protein from the cyanobacterium Synechocystis PCC6803 is a β-1, 3–1, 4-glucanase and functions in salt stress telerance. Biochem J 405:139–146
Wu SC, Ham KS, Darvill AG, Albersheim P (1997) Deletion of two endo-β-1, 4-xylanase genes reveals additional isozymes secreted by the rice blast fungus. Mol Plant-Microb Interact 10:700–708
Acknowledegments
This work was supported in part by the New Energy and Industrial Technology Development Organization of Japan (NEDO).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takeda, T., Takahashi, M., Nakanishi-Masuno, T. et al. Characterization of endo-1,3–1,4-β-glucanases in GH family 12 from Magnaporthe oryzae . Appl Microbiol Biotechnol 88, 1113–1123 (2010). https://doi.org/10.1007/s00253-010-2781-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2781-2