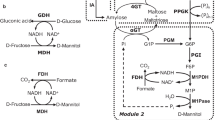
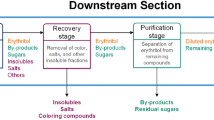
Abstract
Erythritol, a four-carbon polyol, is a biological sweetener with applications in food and pharmaceutical industries. It is also used as a functional sugar substitute in special foods for people with diabetes and obesity because of its unique nutritional properties. Erythritol is produced by microbial methods using mostly osmophilic yeasts and has been produced commercially using mutant strains of Aureobasidium sp. and Pseudozyma tsukubaensis. Due to the high yield and productivity in the industrial scale of production, erythritol serves as an inexpensive starting material for the production of other sugars. This review focuses on the approaches for the efficient erythritol production, strategies used to enhance erythritol productivity in microbes, and the potential biotechnological applications of erythritol.

Similar content being viewed by others
References
Aoki M, Pastore G, Park Y (1993) Microbial transformation of sucrose and glucose to erythritol. Biotechno Lett 15:383–388
Bell A, Wheeler M (1986) Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol 24:411–451
Bernt W, Borzelleca J, Flamm G, Munro I (1996) Erythritol: a review of biological and toxicological studies. Regul Toxicol Pharmacol 24:191
Bilanx M, Flourie B, Jaequemmim C, Messing B (1991) Sugar alcohols. Handbook of Sweeteners. Glasgow, Blackie Academic & Professional, p 72.
Braun M, Niederpruem D (1969) Erythritol metabolism in wild-type and mutant strains of Schizophyllum commune. J Bacteriol 100:625
Brown AD (1978) Compatible solutes and extreme water stress in eukaryotic microorganisms. Adv Microb Physiol 17:181–242
Cerestar Holding BV (1999) Application for assessment of erythritol prior to its authorization. Mitubishi Chemical Corporation and Nikken Chemicals Co. Ltd.
Chu N, Ballou C (1961) The synthesis and properties of d-glycero-tetrulose 1-phosphate and 4-phosphate (d-erythrulose 1-phosphate and 4-phosphate). J Am Chem Soc 83:1711–1715
de Cock P (1999) Erythritol: a novel noncaloric sweetener ingredient. Low-Calorie Sweeteners: Present and Future 85:110–116
de Vries W (1966) Carbohydrate metabolism of Bifidobacterium bifidum. Antonie van Leeuwenhoek 32:452–452
den Hartog G, Boots A, Adam-Perrot A, Brouns F, Verkooijen I, Weseler A, Haenen G, Bast A (2009) Erythritol is a sweet antioxidant. Nutrition. doi:10.1016/j.nut.2009.05.004
Dijkema C, Kester H, Visser J (1985) 13C NMR studies of carbon metabolism in the hyphal fungus Aspergillus nidulans. Proc Natl Acad Sci USA 82:14–18
Frost and Sullivan (2007) Strategic analysis of the erythritol market. In: Frost & Sullivan (eds) Strategic Analysis of the U.S. Polyols Markets.
Goldberg I (1994) Functional foods: designer foods, pharmafood, nutraceuticals. Chapman & Hall, New York, N Y
Goldberg M, Racker E (1962) Formation and isolation of a glycolaldehyde-phosphoketolase intermediate. J Biol Chem 237:3841–3842
Goossens J, Roper H (1994) Erythritol: a new sweetener. Confectionery Production (United Kingdom) 24:182–188
Goossens J, Gonze M (1996) Nutritional properties and applications of erythritol: a unique combination? In: Grenby TH (ed) Advances in sweeteners. Blackie A&P, Bodmin, pp 150–186
Greenley D, Smith D (1979) A novel pathway of glucose catabolism in Thiobacillus novellus. Arch Microbiol 122:257–261
Hajny G, Smith J, Garver J (1964) Erythritol production by a yeastlike fungus. Appl Environ Microbiol 12:240–246
Heath E, Hurwitz J, Horecker B, Ginsburg A (1958) Pentose fermentation by Lactobacillus plantarum. I. The cleavage of xylulose 5-phosphate by phosphoketolase. J Biol Chem 231:1009–1029
Hiele M, Ghoos Y, Rutgeerts P, Vantrappen G (1993) Metabolism of erythritol in humans: comparison with glucose and lactitol. Br J Nutr 69:169–176
Hirata Y, Igarashi K, Ezaki S, Atomi H, Imanaka T (1999) High-level production of erythritol by strain 618A-01 isolated from pollen. J Biosci Bioeng 87:630–635
Holzer H, Schroter W (1962) Zum wirkungsmechanismus der phosphoketolase I. Oxydation verschiedener substrate mit ferricyanid. Biochim Biophys Acta 65:271–288
Horecker BL, Mehler AH (1955) Carbonydrate metabolism. Annu Rev Biochem 24:207–272
Hurwitz J (1958) Pentose phosphate cleavage by Leuconostoc mesenteroides. Biochim Biophys Acta 28:599–602
Ishizuka H, Tokuokak K, Sasaki T, Taniguchi H (1992) Purification and some properties of an erythrose reductase from an Aureobasidium sp. mutant. Biosci Biotechnol Biochem 56:941–945
Ishizuka H, Wako K, Kasumi T, Sasaki T (1989) Breeding of a mutant of Aureobasidium sp. with high erythritol production. J Ferment Bioeng 68:310–314
Jeya M, Lee KM, Tiwari MK, Kim JS, Gunasekaran P, Kim SY, Kim IW, Lee JK (2009) Isolation of a novel high erythritol-producing Pseudozyma tsukubaensis and scale-up of erythritol fermentation to industrial level. Appl Microbiol Biotechnol 83:225–231
Kandler O (1983) Carbohydrate metabolism in lactic acid bacteria. Antonie van Leeuwenhoek 49:209–224
Kasumi T, Sasaki T, Taki A, Nakayama K, Oda T, Wako K (1998) Development of erythritol fermentation and its applications. J Appl Glycosci 45:131–136
Kim KA, Noh BS, Lee JK, Kim SY, Park YC, Oh DK (2000) Optimization of culture conditions for erythritol production by Torula sp. J Microbiol Biotechnol 10:69–74
Kim SY, Lee KH, Kim JH, Oh DK (1997) Erythritol production by controlling osmotic pressure in Trigonopsis variabilis. Biotechnol Lett 19:727–729
Koh ES, Lee TH, Lee DY, Kim HJ, Ryu YW, Seo JH (2003) Scale-up of erythritol production by an osmophilic mutant of Candida magnoliae. Biotechnol Lett 25:2103–2105
Koutinas A, Wang R, Webb C (2007) The biochemurgist-bioconversion of agricultural raw materials for chemical production. Biotechnol Biofuels 1:24–38
Lee DY, Park YC, Kim HJ, Ryu YW, Seo JH (2003a) Proteomic analysis of Candida magnoliae strains by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 3:2330–2338
Lee JK, Ha SJ, Kim SY, Oh DK (2000) Increased erythritol production in Torula sp. by Mn2+ and Cu2+. Biotechnol Lett 22:983–986
Lee JK, Ha SJ, Kim SY, Oh DK (2001) Increased erythritol production in Torula sp. with inositol and phytic acid. Biotechnol Lett 23:497–500
Lee JK, Koo BS, Kim SY (2002a) Fumarate-mediated inhibition of erythrose reductase, a key enzyme for erythritol production by Torula corallina. Appl Environ Microbiol 68:4534–4538
Lee KH, Seo JH, Ryu YW (2002b) Fermentation characteristics of salt-tolerant mutant, Candida magnoliae M26, for the production of erythritol. Korean J Biotechnol Bioeng 17:509–514
Lee JK, Jung HM, Kim SY (2003b) 1, 8-dihydroxynaphthalene (DHN)-melanin biosynthesis inhibitors increase erythritol production in Torula corallina, and DHN-melanin inhibits erythrose reductase. Appl Environ Microbiol 69:3427–3434
Lee JK, Kim SY, Ryu YW, Seo JH, Kim JH (2003c) Purification and characterization of a novel erythrose reductase from Candida magnoliae. Appl Environ Microbiol 69:3710–3718
Lee KJ, Lim JY (2003) Optimized conditions for high erythritol production by Penicillium sp. KJ-UV29, mutant of Penicillium sp. KJ81. Biotechnol Bioprocess Eng 8:173–178
Li G, Otani T, Fujita H, Tajima H, Kawakami H (2005) Polyol polymers, meso-erythritol polymers and their aliphatic acid esters and aliphatic ethers US Patent 6838544.
Lin S, Wen C, Huang C, Chu W. (2002). Erythritol-producing moniliella strains. US Patent 20030008378A1.
Livesay G (2001) Tolerance of low-digestible carbohydrates; a general view. Br J Nutr 85(1):S7–S16
Makinen K, Isotupa K, Kivilompolo T, Makinen P, Toivanen J, Soderling E (2001) Comparison of erythritol and xylitol saliva stimulants in the control of dental plaque and mutans Streptococci. Caries Res 35:129–135
Maret W, Auld D (1988) Purification and characterization of human liver sorbitol dehydrogenase. Biochemistry 27:1622–1628
Mizanur RMD, Takeshita K, Moshino H, Takada G, Izumori K (2001) Production of l-erythrose via l-erythrulose from erythritol using microbial and enzymatic reactions. J Biosci Bioengi 92:237–241
Munro C, Bernt WO, Borzelleca JF, Flamm G, Lynch BS, Kennepohl E, Bär EA, Modderman J (1998) Erythritol: an interpretive summary of biochemical, metabolic, toxicological and clinical data. Food and Chemical Toxicol 36:1139–1174
Oh DK, Cho CH, Lee JK, Kim SY (2001) Increased erythritol production in fed-batch cultures of Torula sp. by controlling glucose concentration. J Ind Microbiol Biotechnol 26:248–252
Onish H, Saito S (1959) Polyalcohols in soy sauce. Hakko Kogaku Zasshi 37:457–461
Onishi H (1960) Studies on osmophilic yeasts. Part IX. Isolation of a new obligate halophilic yeast and some consideration on halophilism. Bull Agric Chem Soc Jpn 24:226–230
Ookura T, Azuma K, Isshiki K, Taniguchi H, Kasumi T, Kawamura Y (2005) Primary structure analysis and functional expression of erythrose reductases from erythritol-producing fungi (Trichosporonoides megachiliensis SNG-42). Biosci Biotechnol Biochem 69:944–951
Papanikolaou S, Fakas S, Fick M, Chevalot I, Galiotou-Panayotou M, Komaitis M, Marc I, Aggelis G (2008) Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: Production of 1, 3-propanediol, citric acid and single cell oil. Bioenergy Res 32:60–71
Park JB, Seo BC, Kim JR, Park YK (1998) Production of erythritol in fed-batch cultures of Trichosporon sp. J Ferment Bioeng 86:577–580
Park YK, Koo MH, Oliveira IM (1996) Biochemical characteristics of osmophilic yeasts isolated from pollens and honey. Biosci Biotechnol Biochem 60:1872–1873
Pavlenko G, Loitsyanskaya M, Nemirovskaya N (1981) The melanin pigment of gluconobacter oxydans. Mol Gen Microbiol Virol 50:718–722
Pfeifer V, Sohns V, Conway H, Lancaster E, Dabic S, Griffin E (1960) Two stage process for dialdehyde starch using electrolytic regeneration of periodic acid. Ind Eng Chem Res 52:201–206
Racker E (1962) Fructose-6-phosphate phosphoketolase from Acetobacter xylinum. Methods Enzymol 5:276–280
Rymowicz W, Rywinska A, Arowska B, Juszczyk P (2006) Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem Pap 60:391–394
Rymowicz W, Rywinska A, Gładkowski W (2008) Simultaneous production of citric acid and erythritol from crude glycerol by Yarrowia lipolytica Wratislavia K1. Chem Pap 62:239–246
Rymowicz W, Rywinska A, Marcinkiewicz M (2009) High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol Lett 31:377–380
Ryu YW, Park CY, Park JB, Kim SY, Seo JH (2000) Optimization of erythritol production by Candida magnoliae in fed-batch culture. J Ind Microbiol Biotechnol 25:100–103
Sawada K, Taki A, Nakano S, Asaba E, Maehara T (2002) Scale-up of erythritol continuous culture. Program and Abstract fo the annual meeting of the Japan Society for Bioscience, Biochemistry and Agrochemistry 3-2 Da-05.
Sawada K, Taki A, Yamakawa T, Seki M (2009) Key role for transketolase activity in erythritol production by Trichosporonoides megachiliensis SN-G42. J Biosci Bioeng 108:385–390
Schramm M, Klybas V, Racker E (1958) Phosphorolytic cleavage of fructose-6-phosphate by fructose-6-phosphate phosphoketolase from Acetobacter xylinum. J Biol Chem 233:1283
Seo JH, Ryu YW, Jung SR, Kim SY (2001). September 2001. Fermentation processes for preparing erythritol by a high salt tolerant mutant of Candida sp. US Patent 6287830B1.
Sgorbati B, Lenaz G, Casalicchio F (1976) Purification and properties of two fructose-6-phosphate phosphoketolases in Bifidobacterium. Antonie Van Leeuwenhoek 42:49–57
Shindou T, Sasaki Y, Eguchi T, Euguchi T, Hagiwara K, Ichikawa T (1989) Identification of erythritol by HPLC and GC-MS and quantitative measurement in pulps of various fruits. J Agric Food Chem 37:1474–1476
Shindou T, Sasaki Y, Miki H, Eguchi T, Hagiwara K, Ichikawa T (1988) Determination of erythritol in fermented foods by high performance liquid chromatography. Shokuhin Eiseigaku Zasshi 29(6):419–422
Tokuoka K, Ishizuka H, Wako K, Taniguchi H (1992) Comparison of three forms of erythrose reductase from an Aureobasidium sp. mutant. J Gen Appl Microbiol 38:145–155
Van Acker S, Van Den Berg D, Tromp M, Griffioen D, Van Bennekom W, Van Der Vijgh W, Bast A (1996) Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med 20:331–342
Veiga-Da-Cunha M, Firme P, San Romao M, Santos H (1992) Application of 13C nuclear magnetic resonance to elucidate the unexpected biosynthesis of erythritol by Leuconostoc oenos. Appl Environ Microbiol 58:2271
Veiga-da-Cunha M, Santos H, Van Schaftingen E (1993) Pathway and regulation of erythritol formation in Leuconostoc oenos. J Bacteriol 175:3941
Wheeler M (1982) Melanin biosynthesis in Verticillium dahliae: dehydration and reduction reactions in cell-free homogenates [Fungi, reactions to fungicides]. Exp Mycol 6(2):171–179
Wheeler M, Stipanovic R (1985) Melanin biosynthesis and the metabolism of flaviolin and 2-hydroxyjuglone in Wangiella dermatitidis. Arch Microbiol 142:234–241
Yang SW, Park JB, Han NS, Ryu YW, Seo JH (1999) Production of erythritol from glucose by an osmophilic mutant of Candida magnoliae. Biotechnol Lett 21:887–890
Yokozawa T, Kim H, Cho E (2002) Erythritol attenuates the diabetic oxidative stress through modulating glucose metabolism and lipid peroxidation in streptozotocin-induced diabetic rats. J Agric FoodChem 50:5485–5489
Yoshida H, Hayashi J, Sugahara T (1986) Studies on free sugars, free sugar alcohols and organic acids of wild mushrooms. J Japan Soc Food Sci Technol (Japan) 33(6):426–433
Yoshida H, Sugahara T, Hayashi J (1984) Free sugars and free sugar alcohols of mushrooms. Nippon Shokuhin Kogyo Gakkashi 31(12):765–771
Acknowledgements
This work was supported by the 21C Frontier Microbial Genomics and Applications Center Program, Ministry of Education, Science & Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hee-Jung Moon and Marimuthu Jeya equally contributed to this work.
Rights and permissions
About this article
Cite this article
Moon, HJ., Jeya, M., Kim, IW. et al. Biotechnological production of erythritol and its applications. Appl Microbiol Biotechnol 86, 1017–1025 (2010). https://doi.org/10.1007/s00253-010-2496-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2496-4