Abstract
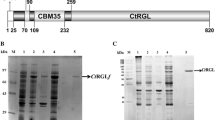
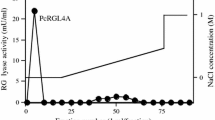
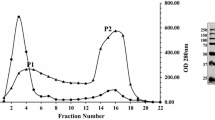
A novel rhamnogalacturonase (RGase) acting on an acetylated substrate was detected in the commercial preparation Driselase, an enzymatic mixture derived from the basidiomycete Irpex lacteus. The activity was isolated by hydrophobic interaction chromatography, gel filtration, and preparative isoelectric focusing, resulting in the isolation of five different rhamnogalacturonan hydrolases exhibiting various isoelectric points from 6.2 to 7.7. Sodium dodecyl sulfate polyacrylamide gel electrophoresis and mass spectrometry analyses after trypsin cleavage of the five fractions revealed that the five rhamnogalacturonases have a molar mass of 55 kDa without any divergences in the identified peptides. The RGase with a pI of 7.2 exhibited a pH optimum between 4.5 and 5 and a temperature optimum between 40°C and 50°C. Its mode of action was analyzed by mass spectrometry of the oligosaccharides produced after hydrolysis of acetylated and nonacetylated rhamnogalacturonan. Oligomers esterified by an acetyl group on the reducing galacturonic acid residue or fully acetylated were detected in the hydrolysate showing that the novel enzyme is able to bind acetylated galacturonic acid in its active site.







Similar content being viewed by others
References
André-Leroux G, Tessier D, Bonnin E (2005) Endopolygalacturonases reveal molecular features for processivity pattern and tolerance towards acetylated pectin. Biochim Biophys Acta Proteins Proteomics 1749:53–64
Axelos MAV, Thibault J-F (1991) Influence of the substituents carboxyl and of the rhamnose content on the solution properties and flexibility of pectins. Int J Biol Macromol 13:77–82
Bobin-Dubigeon C, Hoebler C, Lognoné V, Dagorn-Scaviner C, Mabeau S, Barry J-L, Lahaye M (1997) Chemical composition, physico-chemical properties, enzymatic inhibition and fermentative characteristics of dietary fibres from edible seaweeds. Sci Aliment 17:619–639
Bonnin E, Brunel M, Gouy Y, Lesage-Meessen L, Asther M, Thibault J-F (2001) Aspergillus niger I-1472 and Pycnoporus cinnabarinus MUCL39533, selected for the biotransformation of ferulic acid to vanillin, are also able to produce cell wall polysaccharide-degrading enzymes and feruloyl esterases. Enza Microb Technol 28:70–80
Bonnin E, Clavurier K, Daniel S, Kauppinen S, Mikkelsen JDM, Thibault J-F (2008) Pectin acetylesterases from Aspergillus are able to deacetylate homogalacturonan as well as rhamnogalacturonan. Carbohydr Polym 74:411–418
Bradford MMA (1976) A rapid and sensitive method for the quantitation of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–255
Brown JA, Fry SC (1993) Novel O-D-galacturonoyl esters in the pectic polysaccharides of suspension-cultured plant cells. Plant Physiol 103:993–999
Colquhoun IJ, de Ruiter GA, Schols HA, Voragen AGJ (1990) Identification by n.m.r. spectroscopy of oligosaccharides obtained by treatment of the hairy regions of apple pectin with rhamnogalacturonase. Carbohydr Res 206:131–144
Devouge V, Rogniaux H, Nesi N, Tessier D, Gueguen J, Larre C (2007) Differential proteomic analysis of four near-isogenic Brassica napus varieties bred for their erucic acid and glucosinolate contents. J Proteome Res 6:1342–1353
Domon B, Costello CE (1988) A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjugate 5:397–409
Duquenne B, Eeckhaut T, Werbrouck S, Van Huylenbroeck J (2007) Effect of enzyme concentrations on protoplast isolation and protoplast culture of Spathiphyllum and Anthurium. Plant Cell Tissue Organ Cult 91:165–173
Englyst HN, Cummings JH (1988) Improved method of measurement of dietary fiber as non-starch polysaccharides in plant foods. J Ass Off Anal Chem 71:808–814
Fu J, Prade R, Mort A (2001) Expression and action pattern of Botryotinia fuckeliana (Botrytis cinerea) rhamnogalacturonan-hydrolase in Pichia pastoris. Carbohydr Res 330:73–81
Gardner SL, Burrell MM, Fry SC (2002) Screening of Arabidopsis thaliana stems for variation in cell wall polysaccharides. Phytochemical 60:241–254
Hamada N, Fuse N, Shimosaka M, Kodaira R, Amano Y, Kanda T, Okazaki M (1999a) Cloning and characterization of a new exo-cellulase gene, cel3, in Irpex lacteus. FEMS Microbiol Lett 172:231–237
Hamada N, Hirohashi K (2000) Cloning and transcriptional analysis of exocellulase I gene from Irpex lacteus. J Tokyo Univ Fish 87:39–44
Hamada N, Okumura R, Fuse N, Kodaira R, Shimosaka M, Kanda T, Okazaki M (1999b) Isolation and transcriptional analysis of a cellulase gene (cell) from the basidiomycete Irpex lacteus. J Biosci Bioeng 87:97–102
Hoebler C, Brillouet J-M (1984) Purification and properties of an endo (1→4)-β-d-xylanase from Irpex lacteus (Polyporus tulipiferae). Carbohydr Res 128:141–155
Ishii T (1995a) Isolation and charaterization of acetylated rhamnogalacturonan oligomers liberated from bamboo shoot cell-walls by Driselase. Mokusai Gakkaishi 41:561–572
Ishii T (1995b) Pectic polysaccharides from bamboo shoot cell-walls. Mokuzai Gakkaishi 41:669–676
Kanda T, Amano Y, Nisizawa K (1985) Purification and properties of two endo-1, 4-ß-xylanases from Irpex lacteus (Polyporus tulipiferae). J Biochem 98:1545–1554
Kanda T, Wakabayashi K, Nisizawa K (1976) Xylanase activity of an endo-cellulase of carboxymethylcellulase type from Irpex lacteus (Polyporus tulipiferae). J Biochem 79:989–995
Kofod LV, Kauppinen S, Christgau S, Andersen LN, Heldt-Hansen HP, Dörreich K, Dalbøge H (1994) Cloning and characterization of two structurally and functionally divergent rhamnogalacturonases from Aspergillus aculeatus. J Biol Chem 269:29182–29189
Lahaye M, Thibault JF (1990) Purification of arabinanases and galactanases from Aspergillus niger. In: Proceedings of the 3rd International Workshop on Plant Polysaccharides, Structure, and Function, Le Croisic, France, 19–21 September
Lahaye M, Vigouroux J, Thibault JF (1991) Endo-β-1,4-D-galactanases from Aspergillus niger var. aculeatus. Purification and some properties. Carbohydr Polym 15:431–444
Levigne S, Thomas M, Ralet M-C, Quéméner B, Thibault J-F (2002) Determination of the degrees of methylation and acetylation of pectins using a C18 column and internal standards. Food Hydrocoll 16:547–550
Mutter M, Beldman G, Schols HA, Voragen AGJ (1994) Rhamnogalacturonan α-L-rhamnopyranohydrolase: a novel enzyme specific for the terminal non reducing rhamnosyl unit in rhamnogalacturonan region of pectins. Plant Physiol 106:241–250
Needs PW, Rigby NM, Colquhoun IJ, Ring SG (1998) Conflicting evidence for non-methyl galacturonoyl esters in Daucus carota. Phytochemical 48:71–77
Nelson N (1944) A photometric adaptation of the Somogyi method for determination of glucose. J Biol Chem 153:375–380
Quémener B, Cabrera-Pino JC, Ralet M-C, Bonnin E, Thibault J-F (2003) Assignment of acetyl groups to O-2 and/or O-3 of pectic oligogalacturonides using negative electrospray ionization ion trap mass spectrometry. J Mass Spectrom 38:641–648
Ralet M-C, Crépeau M-J, Bonnin E (2008) Evidence for a blockwise distribution of acetyl groups onto homogalacturonans from a commercial sugar beet (Beta vulgaris) pectin. Phytochemical 69:1903–1909
Schols HA, Geraeds CCJM, Searle-van Leeuwen MF, Kormelink FJM, Voragen AGJ (1990) Rhamnogalacturonase: a novel enzyme that degrades the hairy regions of pectins. Carbohydr Res 206:105–115
Schols HA, Voragen AGJ (1994) Occurrence of pectic hairy regions in various plant cell wall materials and their degradability by rhamnogalacturonase. Carbohydr Res 256:83–95
Schols HA, Voragen AGJ (1996) Complex pectins: structure elucidation using enzymes. In: Visser J, Voragen AGJ (eds) Pectins and pectinases, progress in biotechnology, vol 14. Elsevier, Amsterdam, pp 3–19
Shevchenko A, Sunyaev S, Loboda A, Shevchenko A, Bork P, Ens W, Standing KG (2001) Charting the proteomes of organisms with unsequenced genomes by MALDI-quadrupole time-of-flight mass spectrometry and BLAST homology searching. Anal Chem 73:1917–1926
Sturgeon RJ (1990) Monosaccharides. In: Dey PM, Harbone JB (eds) Methods in plant biochemistry, vol 2. Academic, London, pp 1–37
Suykerbuyk MEG, Kester HCM, Schaap PJ, Stam H, Musters W, Visser J (1997) Cloning and characterization of two rhamnogalacturonases from Aspergillus niger. Appl Environ Microbiol 63:2507–2515
Thibault J-F (1979) Automatisation du dosage des substances pectiques par la méthode au méta-hydroxydiphenyl. Lebensm Wiss Technol 12:247–251
Toda H, Takada S, Oda M, Amano Y, Kanda T, Okazaki M, Shimosaka M (2005) Gene cloning of an endoglucanase from the Basidiomycete Irpex lacteus and its cDNA expression in Saccharomyces cerevisiae. Biosci Biotech Biochem 69:1262–1269
Tsumuraya Y, Mochizuki N, Hashimoto Y, Kovac P (1990) Purification of an exo-β-(1, 3)-D-galactanase of Irpex lacteus (Polyporus tulipiferae) and its action on arabino-galactan-proteins. J Biol Chem 265:7207–7215
Voragen AGJ, Pilnik W, Thibault JF, Axelos MAV, Renard CMGC (1995) Pectins. In: Stephen AM (ed) Food polysaccharides and their applications. Marcel Dekker, New York, pp 287–339
Voragen AGJ, Rombouts FM, Pilnik W (1971) The influence of the degree of esterification on the activity of pectin- and pectate-lyases. Lebensm Wiss Technol 4:126–128
Acknowledgments
We gratefully thank Audrey Geairon and David Ropartz from the ‘‘Biopolymers-Interaction-Structural Biology” platform located at the INRA Center of Nantes (http://www.nantes.inra.fr/plateformes_et_plateaux_techniques/plateforme_bibs) for the mass spectrometry analyses. Sylviane Daniel is acknowledged for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Normand, J., Ralet, MC., Thibault, JF. et al. Purification, characterization, and mode of action of a rhamnogalacturonan hydrolase from Irpex lacteus, tolerant to an acetylated substrate. Appl Microbiol Biotechnol 86, 577–588 (2010). https://doi.org/10.1007/s00253-009-2310-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2310-3