Abstract
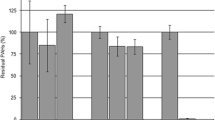
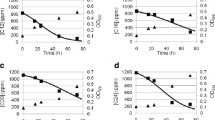
The biocatalytic generation of high-value chemicals from abundant, cheap and renewable feedstocks is an area of great contemporary interest. A strain of Rhodococcus erythropolis designated MLT1 was isolated by selective enrichment from the soil surrounding hop plants, using the abundant triene β-myrcene from hops as a sole carbon source for growth. Resting cells of the organism were challenged with β-myrcene, and the major product of biotransformation was determined by mass spectrometric analysis to be the monoterpene alcohol geraniol. Controls demonstrated that the product was biogenic and that an aerobic environment was required. The ability to transform β-myrcene was shown to be restricted to cells that had been grown on this substrate as sole carbon source. Pre-incubation of cells with the cytochrome P450 inhibitors metyrapone or 1-aminobenzotriazole reduced geraniol production by 23% and 73% respectively, but reduction in activity was found not to correlate with the inhibitor concentration. A comparative analysis of insoluble and soluble cell extracts derived from cells of MLT1 grown on either β-myrcene or glucose revealed at least four proteins that were clearly overproduced in response to growth on β-myrcene. Mass spectrometric analysis of tryptic digests of three of these protein bands suggested their identities as an aldehyde dehydrogenase, an acyl-CoA dehydrogenase and a chaperone-like protein, each of which has a precedented role in hydrocarbon metabolism clusters in Rhodococcus sp. and which may therefore participate in a β-myrcene degradation pathway in this organism.





Similar content being viewed by others
References
Adams RP (1995) Identification of essential oil components by gas chromatography – mass spectrometry. Allured, IL
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Busmann D, Berger RG (1994) Conversion of myrcene by submerged cultured basidiomycetes. J Biotechnol 37:39–43
Chen ZM, Liu JH, Tao JH (2007) Biocatalysis for green chemistry and drug development. Prog Chem 19:1919–1927
Cheng AX, Lou YG, Mao YB, Lu S, Wang LJ, Chen XY (2007) Plant terpenoids: biosynthesis and ecological functions. J Integr Plant Biol 49:179–186
de Carvalho C, da Fonseca MMR (2003) Towards the bio-production of trans-carveol and carvone from limonene: induction after cell growth on limonene and toluene. Tetrahedron Asymmetr 14:3925–3931
de Carvalho C, da Fonseca MMR (2006) Carvone: why and how should one bother to produce this terpene. Food Chem 95:413–422
de Oliveira A, Ribiero-Pinto L, Otto S (1997) Induction of liver monooxygenases by beta-myrcene. Toxicology 124:135–140
Duetz WA, Bouwmeester H, van Beilen JB, Witholt B (2003) Biotransformation of limonene by bacteria, fungi, yeasts, and plants. Appl Microbiol Biotechnol 61:269–277
Ensign SA (2001) Microbial metabolism of aliphatic alkenes. Biochemistry 40:5845–5853
Farooq AR, Rahman A, Choudhary AI (2004) Fungal transformation of monoterpenes. Curr Org Chem 8:353–367
Förster-Fromme K, Chattopadhyay A, Jendrossek D (2008) Biochemical characterization of AtuD from Pseudomonas aeuruginosa, the first member of a new subgroup of acyl-CoA dehydrogenases for citronellyl-CoA. Microbiology 154:789–796
Förster-Fromme K, Höschle B, Mack C, Bott M, Armbruster W, Jendrossek D (2006) Identification of genes and proteins necessary for catabolism of acyclic terpenes and leucine/isovalerate in Pseudomonas aeuruginosa. Appl Environ Microbiol 72:4819–4828
Foβ S, Harder J (1997) Microbial transformation of a tertiary allylalcohol: regioselective isomerisation of linalool to geraniol without nerol formation. FEMS Microbiol Lett 149:71–75
Howell AR, Pattenden G (1990a) Hydrocobaltation reactions of 1, 3-dienes - regioselective hydroxylation of myrcene to geraniol and to (+/-)-linalool via allylcobaloxime intermediates. J Chem Soc, Perkin Trans 1:2715–2719
Howell AR, Pattenden G (1990b) Regioselective hydroxylations of 1, 3-dienes via hydrocobaltation reactions—facile conversion of myrcene to geraniol and to (+/−)-linalool. J Chem Soc Chem Commun 2:103–104
Ishida T (2005) Biotransformation of terpenoids by mammals, microorganisms, and plant-cultured cells. Chem Biodivers 2:569–590
Iurescia S, Marconi AM, Tofani D, Gambacorta A, Paterno A, Devirgiliis C, van der Werf MJ, Zennaro E (1999) Identification and sequencing of beta-myrcene catabolism genes from Pseudomonas sp strain M1. Appl Environ Microbiol 65:2871–2876
Jennings W, Shibamoto T (1980) Qualitative analysis of flavour and fragrance volatiles by glass capillary gas chromatography. Academic, New York
Kang HK, Lee SS (1997) Heterogeneous natures of the microbial steroid 9α-hydroxylase in nocardioforms. Arch Pharm Res 20:519–524
Kishimoto T, Wanikawa A, Kagami N, Kawatsura K (2005) Analysis of hop-derived terpenoids in beer and evaluation of their behavior using the stir bar-sorptive extraction method with GC-MS. J Agric Food Chem 53:4701–4707
Krings U, Berger RG (1998) Biotechnological production of flavours and fragrances. Appl Microbiol Biotechnol 49:1–8
Krings U, Hapetta D, Berger RG (2008) A labeling study on the formation of perillene by submerged cultured oyster mushroom, Pleurotus ostreatus. Appl Microbiol Biotechnol 78:533–541
Krugener S, Schaper C, Krings U, Berger RG (2009) Pleurotus species convert monoterpenes to furanoterpenoids through 1, 4-endoperoxides. Biores Technol 100:2855–2860
Madyastha KM (1987) Metabolism of beta-myrcene in-vivo and in-vitro - its effects on rat-liver microsomal-enzymes. Xenobiotica 17:539–549
Miyazawa M, Murata T (2000) Biotransformation of beta-myrcene by the larvae of common cutworm (Spodoptera litura). J Agric Food Chem 48:123–125
Ojala M, Ketola RA, Mansikka T, Kotiaho T, Kostiainen R (1999) Determination of mono- and sesquiterpenes in water samples by membrane inlet mass spectrometry and static headspace gas chromatography. Talanta 49:179–188
Panke S, Witholt B, Schmid A, Wubbolts MG (1998) Towards a biocatalyst for (S)-styrene oxide production: characterization of the styrene degradation pathway of Pseudomonas sp. strain VLB120. Appl Environ Microbiol 64:2032–2043
Rocha SM, Coelho E, Zrostlikova J, Delgadillo I, Coimbra MA (2007) Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry of monoterpenoids as a powerful tool for grape origin traceability. J Chromatogr A 1161:292–299
Rozenbaum HF, Patitucci ML, Antunes OAC, Pereira N (2006) Production of aromas and fragrances through microbial oxidation of monoterpenes. Br J Chem Eng 23:273–279
Sandstrom P, Welch WH, Blomquist GJ, Tittiger C (2006) Functional expression of a bark beetle cytochrome P450 that hydroxylates myrcene to ipsdienol. Insect Biochem Mol Biol 36:835–845
Savithiry N, Cheong TK, Oriel P (1997) Production of alpha-terpineol from Escherichia coli cells expressing thermostable limonene hydratase. Appl Biochem Biotechnol 63–65:213–220
Schwab W, Davidovich-Rikanati R, Lewinsohn E (2008) Biosynthesis of plant-derived flavor compounds. Plant J 54:712–732
Serra S, Fuganti C, Brenna E (2005) Biocatalytic preparation of natural flavours and fragrances. Trends Biotechnol 23:193–198
Sharp JO, Sales CM, LeBlanc JC, Liu J, Wood TK, Eltis LD, Mohn WW, Alvarez-Cohen L (2007) An inducible propane monooxygenase is responsible for N-nitrosodimethylamine degradation by Rhodococcus sp strain RHA1. Appl Environ Microbiol 73:6930–6938
Shevchenko A, Sunyaev S, Loboda A, Shevchenko A, Bork P, Enz W, Standing KG (2001) Charting the proteomes of organisms with unsequenced genomes by MALDI quadrupole time-of-flight mass spectrometry and BLAST homology searching. Anal Chem 73:1917–1926
Small FJ, Ensign SA (1997) Alkene monooxygenase from Xanthobacter strain Py2. J Biol Chem 272:24913–24920
Takahashi M, Suzuki H, Morooka Y, Ikawa T (1979) Regioselective hydroxylation of beta-myrcene using Pd(II) complexes. Chem Lett 8:53–56
Van Hylckama Vlieg JET, Leemhuis H, Lutje Spelberg JH, Janssen DB (2000) Characterisation of the gene cluster involved in isoprene metabolism in Rhodococcus sp. strain AD45. J Bacteriol 182:1956–1963
Vandamme EJ, Soetaert W (2002) Bioflavours and fragrances via fermentation and biocatalysis. J Chem Technol Biotechnol 77:1323–1332
Woodley JM (2008) New opportunities for biocatalysis: making pharmaceutical processes greener. Trends Biotechnol 26:321–327
Yamazaki Y, Hayashi Y, Hori N, Mikami Y (1988) Microbial conversion of β-myrcene by Aspergillus niger. Agric Biol Chem 52:2921–2922
Acknowledgments
We are grateful to Botanix Ltd, Paddock Wood, Kent, U.K. and to the Biotechnology and Biological Sciences Research Council for funding a CASE studentship to M.L.T. We are also grateful to Botanix for providing plant material, chemicals and GC-MS services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, M.L., Marriott, R., Dowle, A. et al. Biotransformation of β-myrcene to geraniol by a strain of Rhodococcus erythropolis isolated by selective enrichment from hop plants. Appl Microbiol Biotechnol 85, 721–730 (2010). https://doi.org/10.1007/s00253-009-2182-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2182-6