Abstract
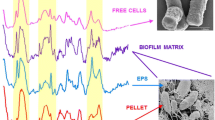
The Raman spectra, water content, and biomass density of wild-type (WT) Pseudomonas aeruginosa PAO1, small colony variant (SCV) PAO1, and Pseudoalteromonas sp. NCIMB 2021 biofilms were compared in order to determine their variation with strain and species. Living, fully submerged biofilms were analyzed in situ by confocal Raman microspectroscopy for up to 2 weeks. Water to biomass ratios (W/BRs), which are the ratios of the O–H stretching vibration of water at 3,450 cm−1 to the C–H stretching band characteristic of biomass at 2,950 cm−1, were used to estimate the biomass density and cell density by comparison with W/BRs of protein solutions and bacterial suspensions, respectively, on calibration curves. The hydration within SCV biofilm colonies was extremely heterogeneous whereas W/BRs were generally constant in young WT biofilm colonies. The mean biomass in biofilm colonies of WT or colony cores of SCV was typically equivalent to 16% to 27% protein (w/v), but was 10% or less for NCIMB 2021. The corresponding cell densities were 7.5 to >10 × 1010 cfu mL−1 for SCV, while the maximum cell density for NCIMB biofilms was 2.8 × 1010 cfu mL−1.







Similar content being viewed by others
References
Aarnoutse PJ, Westerhuis JA (2005) Quantitative Raman reaction monitoring using the solvent as internal standard. Anal Chem 77:1228–1236
Choo-Smith LP, Maquelin K, van Vreeswijk T, Bruining HA, Puppels GJ, Ngo-Thi NA, Kirschner C, Naumann D, Ami D, Villa AM, Orsini F, Doglia SM, Lamfarraj H, Sockalingum DG, Manfait M, Allouch P, Endtz HP (2001) Investigating microbial (micro) colony heterogeneity by vibrational spectroscopy. Appl Environ Microbiol 67:1461–1469
Déziel E, Comeau Y, Villemur R (2001) Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J Bacteriol 183:1195–1204
Everall N (2004a) Depth profiling with confocal Raman microscopy, part I. Spectroscopy 19:22–33
Everall N (2004b) Depth profiling with confocal Raman microscopy, part II. Spectroscopy 19:16–27
Häußler S (2004) Biofilm formation by the small colony variant phenotype of Pseudomonas aeruginosa. Environ Microbiol 6:546–551
Kirisits MJ, Prost L, Starkey M, Parsek MR (2005) Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 71:4809–4821
Maquelin K, Kirschner C, Choo-Smith LP, Ngo-Thi NA, van Vreeswijk T, Stammler M, Endtz HP, Bruining HA, Naumann D, Puppels GJ (2003) Prospective study of the performance of vibrational spectroscopies for rapid identification of bacterial and fungal pathogens recovered from blood cultures. J Clin Microbiol 41:324–329
Marcotte L, Therien-Aubin H, Sandt C, Barbeau J, Lafleur M (2004a) Solute size effects on the diffusion in biofilms of Streptococcus mutans. Biofouling 20:189–201
Marcotte L, Barbeau J, Lafleur M (2004b) Characterization of the diffusion of polyethylene glycol in Streptococcus mutans biofilms by Raman microspectroscopy. Appl Spectrosc 58:1295–1301
Neu TR, Swernhone GDW, Lawrence JR (2001) Assessment of lectin binding analysis for in situ detection of glycoconjugates in biofilms systems. Microbiology 147:299–313
Nivens DE, Ohman DE, Williams J, Franklin MJ (2001) Role of alginate and its O-acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J Bacteriol 183:1047–1057
Pätzold R, Keuntje M, Anders-von Ahlften A (2006) A new approach to non-destructive analysis of biofilms by confocal Raman microscopy. Anal Bioanal Chem 386:286–292
Pelletier MJ (2003) Quantitative analysis using Raman spectrometry. Appl Spectrosc 57:20A–42A
Purevdorj-Gage B, Costerton WJ, Stoodley P (2005) Phenotypic differentiation and seeding dispersal in non-mucoid and mucoid Pseudomonas aeruginosa biofilms. Microbiology 151:1569–1576
Sandt C, Smith-Palmer T, Pink J, Brennan L, Pink DA (2007) Confocal Raman microspectroscopy as a tool for studying the chemical heterogeneities of biofilms in situ. J Appl Microbiol 103:1808–1820
Sandt C, Smith-Palmer T, Pink J, Pink DA (2008) Quantification of local water and biomass in wild type PAO1 biofilms by confocal Raman microspectroscopy. J Microbiol Meth 75:148–152
Schneider M, Mühlemann K, Droz S, Couzinet S, Casaulta C, Zimmerli S (2008) Clinical characteristics associated with isolation of small-colony variants of Staphylococcus aureus and Pseudomonas aeruginosa from respiratory secretions of patients with cystic fibrosis. J Clin Microbiol 46:1832–1834
Staudt C, Horn H, Hempel DC, Neu TR (2004) Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms. Biotechnol Bioeng 88:585–592
Suci PA, Geesey GG, Tyler BJ (2001) Integration of Raman microspectroscopy, differential interference contrast microscopy, and attenuated total reflection Fourier transform infrared spectroscopy to investigate chlorhexidine spatial and temporal distribution in Candida albican. J Microbiol Meth 46:193–208
Webb JS, Lau M, Kjellberg S (2004) Bacteriophage and phenotypic variation in Pseudomonas aeruginosa biofilm development. J Bacteriol 186:8066–8073
Weiner R, Langille S, Quintero E (1995) Structure, function and immunochemistry of bacterial exopolysaccharides. J Ind Microbiol 15:339–346
Acknowledgements
We would like to thank Werner Schnepf for the contributions to the design of the flow cell and for fabricating it. We also thank Leslie Klapstein for her experimental work and Sarah Schooling for her expert advice. We thank the Atlantic Innovation Fund–Atlantic Canada Opportunities Agency, the Natural Sciences and Engineering Research Council, and the St Francis Xavier University Council for Research for the funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandt, C., Smith-Palmer, T., Comeau, J. et al. Quantification of water and biomass in small colony variant PAO1 biofilms by confocal Raman microspectroscopy. Appl Microbiol Biotechnol 83, 1171–1182 (2009). https://doi.org/10.1007/s00253-009-2072-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2072-y