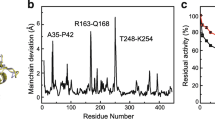
Abstract
Aspergillus niger phytase (PhyA) has been used as a feed supplement to reduce manure phosphorus excretion of swine and poultry but lacks sufficient thermostability for feed pelleting and appropriate pH-activity profile for phytate hydrolysis in the stomach of animals. Previously, a thermostable mutant PhyA18 and two pH-activity profile-improved mutants E228K and K300E were developed. In this study, the mutations were combined to determine if both improvements were cumulative. Four substitutions (S149P, F131L, K112R, and K195R) identified from random mutagenesis were added sequentially to the combined mutants to further improve their thermostability. Mutant E228K shifted the optimum pH of the parent one from 5.5 to 4.0 and increased (P < 0.05) its specific activity at pH 3.5, whereas mutant K300E eliminated the activity dip at pH 3.5 shown in the wild type. Mutant S149P further improved thermostability over PhyA18. Our results illustrate the feasibility and structural basis to improve thermostability and pH-activity profile of PhyA phytase by assembling mutations derived from rational design and random mutagenesis.




Similar content being viewed by others
References
Arnold FH, Wintrode PL, Kentaro M, Gershenson A (2001) How enzymes adapt: lessons from directed evolution. Trends Biochem Sci 26:100–106
Bloom JD, Meyer MM, Meinhold P, Otey CR, MacMillan D, Arnold FH (2005) Evolving strategies for enzyme engineering. Curr Opin Struck Biol 15:447–452
Chen Z, Hong S, Spreitzer RJ (1993) Thermal instability of ribulose-1,5-bisphosphate carboxylase/oxygenase from a temperature-conditional chloroplast mutant of Chlamydomonas reinhardtii. Plant Physiol 101:1189–1194
Cherry JR, Fidantsef AL (2003) Directed evolution of industrial enzymes: an update. Curr Opin Biotech 14:438–443
Declerck N, Machius M, Joyet P, Wiegand G, Huber R, Gaillardin C (2003) Hyperthermostabilization of Bacillus lichniformis a-amylase and modulation of its stability over a 50°C temperature range. Protein Eng 16:287–293
Eilers M, Shekar SC, Shieh T, Smith SO, Fleming PJ (2000) Internal packing of helical membrane proteins. Proc Natl Acad Sci U S A 97:5796–5801
Gentile JM, Roneker KR, Crowe SE, Pond WG, Lei XG (2003) Effectiveness of an experimental consensus phytase in improving dietary phytate-phosphorus utilization by weanling pigs. J Anim Sci 81:2751–2757
Han Y, Lei XG (1999) Role of glycosylation in the functional expression of an Aspergillus niger phytase (PhyA) in Pichia pastoris. Arch Biochem Biophys 364:83–90
Han Y, Wilson DB, Lei XG (1999) Expression of an Aspergillus niger phytase gene (phyA) in Saccharomyces cerevisiae. Appl Environ Microbiol 65:1915–1918
Kim T, Mullaney EJ, Porres JM, Roneker KR, Crowe S, Rice S, Ko T, Ullah AHJ, Daly CB, Welch R, Lei XG (2006) Shifting the pH profile of Aspergillus niger PhyA phytase to match the stomach pH enhances its effectiveness as an animal feed additive. Appl Environ Microbiol 72:4397–4403
Korkegian A, Black ME, Baker D, Stoddard BL (2005) Computational thermostabilization of an enzyme. Science 308:857–860
Kostrewa D, Gruninger-Leitch F, D’Arcy A, Broger C, Mitchell D, van Loon APGM (1997) Crystal structure of phytase from Aspergillus ficuum at 2.5 A resolution. Nat Struct Biol 4:85–190
Kostrewa D, Wyss M, D’Arcy A, van Loon APGM (1999) Crystal structure of Aspergillus niger pH2.5 acid phosphatase at 2.4Å resolution. J Mol Biol 288:965–974
Law GHE, Gandelman OA, Tisi LC, Lowe CR, Murray JAH (2006) Mutagenesis of solvent-exposed amino acids in Photinus pyralis luciferase improves thermostability and pH-tolerance. Biochem J 397:305–312
Leemhuis H, J.Rozeboom H, Dijkstra BW, Dijkhuizen L (2004) Improved thermostability of Bacillus circulans cyclodextrin glycosyltransferase by the introduction of a salt bridge. Proteins 54:128–134
Lei XG, Stahl CH (2001) Biotechnological development of effective phytases for mineral nutrition and environmental protection. Appl Microbiol Biotechnol 57:474–481
Lim D, Golovan S, Forsberg CW, Jia1 Z (2000) Crystal structures of Escherichia coli phytase and its complex with phytate. Nat Struct Biol 7:108–113
Liu H-L, Doleyres Y, M.Coutinho P, Ford C, J.Reilly P (2000) Replacement and deletion mutations in the catalytic domain and belt region of Aspergillus awamori glucoamylase to enhance thermostability. Protein Eng 13:655–659
Liu Q, Huang Q, Lei XG, Hao Q (2004) Crystallographic snapshots of Aspergillus fumigatus phytase, revealing its enzymatic dynamics. Structure 12:1575–1583
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mamdouh Ba, Bassem K, Xavier R, Richard H, Samir B (2006) Thermostability enhancement and change in starch hydrolysis profile of the maltohexaose-forming amylase of Bacillus stearothermophilus US100 strain. Biochem J 394:51–56
Matthews BW, Nicholson H, Becktel W (1987) Enhanced protein thermostability from site-directed mutations that decrease the entropy of unfolding. Proc Natl Acad Sci U S A 84:6663–6667
Mullaney EJ, Daly CB, Ullah AHJ (2000) Advances in phytase research. Adv Appl Microbiol 47:157–199
Mullaney EJ, Daly CB, Kim T, Porres JM, Lei XG, Sethumadhavan K, Ullah AHJ (2002) Site-directed mutagenesis of Aspergillus niger NRRL 3135 phytase at residue 300 to enhance catalysis at pH 4.0. Biochem Biophys Res Commun 297:1016–1020
Nakamura S, Tanaka T, Yada R, Nakai S (1997) Improving the thermostability of Bacillus stearothermophilus neutral protease by introducing proline into the active site helix. Protein Eng 10:1263–1269
Pagano AR, Roneker KR, Lei XG (2007) Distribution of supplemental Escherichia coli AppA2 phytase activity in digesta of various gastrointestinal segments of young pigs. J Anim Sci 85:1444–1452
Santarossa G, Lafranconi PG, Alquati C, DeGioia L, Alberghina L, Fantucci P, Lotti M (2005) Mutations in the “lid” region affect chain length specificity and thermostability of a Pseudomonas fragi lipase. FEBS Lett 579:2383–2386
Sriprapundh D, Vieille C, Zeikus JG (2000) Molecular determinants of xylose isomerase thermal stability and activity: analysis of thermozymes by site-directed mutagenesis. Protein Eng 13:259–265
Stemmer WPC (1994) DNA shuffling by random fragmentation and reassembly: In vitro recombination for molecular evolution. Proc Natl Acad Sci U S A 91:10747–10751
Ullah AHJ, Gibson DM (1987) Extracellular phytase (E.C.3.1.3.8.) from Aspergillus ficuum NRRL3135: purification and characterization. Prep Biochem 17:63–91
Vogt G, Woell S, Argos P (1997) Protein thermal stability, hydrogen bonds, and ion pairs. J Mol Biol 269:631–643
Watanabe K, Masuda T, Ohashi H, Mihara H, Suzuki Y (1994) Multiple proline substitutions cumulatively thermostabilize Bacillus cereus ATCC7064 oligo-1,6-glucosidase. Irrefragable proof supporting the proline rule. Eur J Biochem 226:277–283
Wyss M, Brugger R, Kronenberger A, Remy R, Fimbel R, Oesterhelt G, Lehmann M, van Loon APGM (1999) Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): catalytic properties. Appl Environ Microbiol 65:367–373
Xiang T, Liu Q, Deacon AM, Koshy M, Kriksunov IA, Lei XG, Hao Q, Thiel DJ (2004) Crystal structure of a heat-resilient phytase from Aspergillus fumigatus, carrying a phosphorylated histidine. J Mol Biol 339:437–445
Yasuda K, Roneker KR, Miller DD, Welch RM, Lei XG (2006) Supplemental dietary inulin affects the bioavailability of iron in corn and soybean meal to young pigs. J Nutr 136:3033–3038
Zhang W, Mullaney EJ, Lei XG (2007) Adopting selected hydrogen bonding and ionic interactions from Aspergillus fumigatus phytase structure improves the thermostability of Aspergillus niger PhyA phytase. Appl Environ Microbiol 73:3069–3076
Acknowledgments
This research was supported in part by a Cornell Biotechnology Program grant (to XL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, W., Lei, X.G. Cumulative improvements of thermostability and pH-activity profile of Aspergillus niger PhyA phytase by site-directed mutagenesis. Appl Microbiol Biotechnol 77, 1033–1040 (2008). https://doi.org/10.1007/s00253-007-1239-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1239-7