Abstract
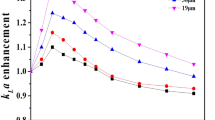
Amberlite XAD-7, a hydrophobic polymer, was used to change microbial reaction of ketones from reduction to Baeyer–Villiger (BV) oxidation. Thus, D. magnusii NBRC 4600 and G. reessii NBRC 1112 could catalyze the BV reaction of ketones in the presence of the polymer while reduction of the substrates proceeded, and BV oxidation was scarcely found in the absence of the polymer.


Similar content being viewed by others
References
Alphand V, Archelas A, Furstoss R (1990) Microbiological transformations. 13. Direct synthesis of both S and R enantiomers of 5-hexadecanolide via an enantioselective microbiological Baeyer–Villiger reaction. J Org Chem 55:347–350
Anderson BA, Hansen MM, Harkness AR, Henry CL, Vicenzi JT, Zmijewski MJ (1995) Application of a practical biocatalytic reduction to an enantioselective synthesis of the 5H-2,3-benzodiazepine LY300164. J Am Chem Soc 117:12358–12359
Britton LN, Brand JM, Markovetz AJ (1974) Source of oxygen in the conversion of 2-tridecanone to undecyl acetate by Pseudomonas cepacia and Nocardia sp. Biochim Biophys Acta 369:45–49
Chapman PJ, Meerman G, Gunsalus IC (1965) The microbiological transformation of fenchone. Biochem Biophys Res Commun 20:104–108
Cripps GE (1975) The microbial metabolism of acetophenone. Metabolism of acetophenone and some chloroacetophenones by an Arthrobacter species. Biochem J 152:233–241
D’ Arrigo P, Fuganti C, Servi S, Strini A (1997) The effect of absorbing resins on substrate concentration and enantiomeric excess in yeast reduction. Tetrahedron Asymmetry 8:2375–2379
D’ Arrigo P, Fuganti C, Fantoni GP, Servi S (1998) Extractive biocatalysis: a powerful tool in selectivity control in yeast biotransformations. Tetrahedron 54:15017–15026
Donoghue NA, Trudgill PW (1975) The metabolism of cyclohexanol by Acinetobacter NCIB 9871. Eur J Biochem 60:1–7
Donoghue NA, Norris DB, Trudgill PW (1976) The purification and properties of cyclohexanone oxygenase from Nocardia globerula CL1 and Acinetobacter NCIB 9871. Eur J Biochem 63:175–192
Gagnon R, Grogan GM, Levitt S, Roberts SM, Wan PWH, Willetts AJ (1994) Biological Baeyer–Villiger oxidation of some monocyclic and bicyclic ketones using monooxygenases from Acinetobacter calcoaceticus NCIMB 9871 and Pseudomonas putida NCIMB 10007. J Chem Soc Perkin Trans 1:2537–2543
Hasegawa H, Hamano K, Obata H, Tokuyama T (1982) Microbial degradation of cycloheptanone. Agric Biol Chem 46:1139–1143
Kayser M, Chen G, Stewart J (1999) “Designer yeast”: an enantioselective oxidizing reagent for organic synthesis. Synlett 1:153–158
Lee SS, Sih CJ (1967) Mechanisms of steroid oxidation by microorganisms. XII. Metabolism of hexahydroindanpropionic acid derivatives. Biochemistry 6:1395–1403
Mahato SB, Banerjee S, Podder S (1987) Novel microbial transformation of 16-dehydro pregnenolone by Arthrobacter simplex. Tetrahedron Lett 28:5315–5318
Mihovilovic MD, Muller B, Stanetty P (2002) Monooxygenase-mediated Baeyer–Villiger oxidations. Eur J Org Chem 2002:3711–3739
Mihovilovic MD, Rudroff F, Winninger A, Schneider T, Schulz F, Reetz MT (2006) Microbial Baeyer–Villiger oxidation: stereopreference and substrate acceptance of cyclohexanone monooxygenase mutants prepared by directed evolution. Org Lett 6:1221–1224
Nakamura K, Kondo S, Kawai Y, Ohno A (1993) Asymmetric reduction of ketopantolactone by baker’s yeast. Tetrahedron Asymmetry 4:1253–1254
Nakamura K, Kondo S, Ohno A (1994) Effect of cyclodextrin on improvement of enantioselectivity in the reduciton of Ketopantolactone with Bakers’ Yeast. Bioorg Med Chem 2:433–437
Nakamura K, Fujii M, Ohno A (1997) Effect of XAD, a solid organic solvent, on microbial reduction. 3rd international symposium on biocatalysis and biotransformation-Biotrans 97’ in France, Abstract pp 84
Nakamura K, Fujii M, Ida Y (2000) Asymmetric reduction of ketones by Geotrichum candidum in the presence of Amberlite XAD, a solid organic solvent. J Chem Soc Perkin Trans 1:3205–3211
Nakamura K, Fujii M, Ida Y (2001) Stereoinversion of arylalcohols by Geotrichum candidum. Tetrahedron Asymmetry 12:3147–3153
Nakamura K, Takenaka K, Fujii M, Ida Y (2002) Asymmetric synthesis of both enantiomers of secondary alcohols by reduction with a single microbe. Tetrahedron Lett 43:3629–3631
Ouazzani-Chahdi J, Buisson D, Azerad R (1987) Preparation of both enantiomers of a chiral lactone through combined microbiological reduction and oxidation. Tetrahedron Lett 28:1109–1112
Ryerson CC, Ballou DP, Walsh C (1982) Mechanistic studies on cyclohexanone oxygenase. Biochemistry 21:2644–2655
Schwab JM, Li W, Thomas LP (1983) Cyclohexanone oxygenase: stereochemistry, enantioselectivity, and regioselectivity of an enzyme-catalyzed Baeyer–Villiger reaction. J Am Chem Soc 105:4800–4808
Simpson HD, Alphand V, Furstoss R (2001) Microbiological transformations: 49. Asymmetric biocatalysed Baeyer–Villiger oxidation: improvement using a recombinant Escherichia coli whole cell biocatalyst in the presence of an adsorbent resin. J Mol Catal B Enzym 16:101–108
Stewart JD, Reed KW, Zhu J, Chen G, Kayse MM (1996) A “designer yeast” that catalyzes the kinetic resolutions of 2-alkyl-substituted cyclohexanones by enantioselective Baeyer–Villiger oxidations. J Org Chem 61:7652–7653
Taschner MJ, Black DJ (1988) The enzymatic Baeyer–Villiger oxidation: enantioselective synthesis of lactones from mesomeric cyclohexanones. J Am Chem Soc 110:6892–6893
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujii, M., Akita, H., Ida, Y. et al. Control of chemoselectivity of microbial reaction with resin adsorbent: enhancement of Baeyer–Villiger oxidation over reduction. Appl Microbiol Biotechnol 77, 45–51 (2007). https://doi.org/10.1007/s00253-007-1146-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1146-y