Abstract
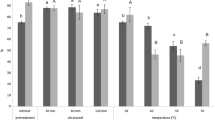
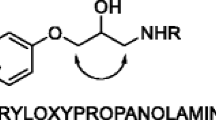
A yeast strain, Candida tropicalis PBR-2, isolated from soil, is capable of carrying out the enantioselective reduction of N,N-dimethyl-3-keto-3-(2-thienyl)-1-propanamine to (S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)-1-propanamine, a key intermediate in the synthesis of the chiral drug (S)-Duloxetine. The organism produced the enantiopure (S)-alcohol with a good yield (>80%) and almost absolute enantioselectivity, with an enantiomeric excess (ee) >99%. Parameters of the bioreduction reaction were optimized and the optimal temperature and pH for the reduction were found to be 30°C and 7.0, respectively. The optimized substrate and the resting cell concentration were 1 g/l and 250 g/l, respectively. The preparative-scale reaction using resting cells of C. tropicalis yielded the (S)-alcohol at 84–88% conversion and ee >99%.






Similar content being viewed by others
References
Asano Y (2002) Overview of screening for new microbial catalysts and their uses in organic synthesis-selection and optimization of biocatalysts. J Biotechnol 94:65–72
Barnett JA, Payne RW, Yarrow D (1983) Yeasts—characteristics and identification. Cambridge University, Cambridge
Berglund RA (1993) Asymmetric synthesis. US patent 5,362,886
Faber K (2000) Biotransformations in organic chemistry, 4th edn. Springer, Berlin Heidelberg New York
Heidlas J, Engel KH, Tressi R (1991) Enantioselectivities of enzymes involved in the reduction of methyl ketones by Bakers’ yeast. Enzyme Microb Technol 13:817–821
Kamal A, Khanna GBR, Ramu R, Krishnaji T (2003) Chemoenzymatic synthesis of duloxetine and its enantiomer:lipase catalysed resolution of 3-hydroxy 3-(2-thienyl) propanenitrile. Tetrahedron Lett 44:4783–4787
Kurtzman CP, Fell JW (1998) The yeasts, a taxonomic study, 4th edn. Elsevier, Amsterdam
Laumen K, Kittelmann M, Ghisalba (2002) Chemoenzymatic approaches for the creation of novel chiral building blocks and reagents for pharmaceutical applications. J Mol Catal B Enzym 19/20:55–66
Liu HL, Hoff BH, Anthonsen T (2000a) Chemo-enzymatic synthesis of the antidepressant duloxetine and its enantiomer. Chirality 12:26–29
Liu HL, Hoff BH, Anthonsen T (2000b) Chemoenzymatic synthesis of antidepressant duloxetine and its enantiomer. J Chem Soc Perkin Trans I 93:1767–1769
Nakamura K, Kawai Y, Nakajima N, Ohno A (1991) Stereochemical control of microbial reduction. A method for controlling the enantioselectivity of reductions with bakers’ yeast. J Org Chem 56:4778–4788
Nakamura K, Yamanaka R, Matsuda T, Harada T (2003) Recent developments in asymmetric reduction of ketones with biocatalysts. Tetrahedron Asymm 14:2659–2681
Ni Y, Hexu J (2002) Asymmetric reduction of aryl ketones with a new isolate Rhodotorula sp. AS 2.2241. J Mol Catal B Enzym 18:233–241
Robertson DW, Wong DT, Krushinski JH (1990) 3-aryloxy-3-substituted propanamines. US patent 5,023,269
Sakai K, Sakurai R, Yuzawa A, Kobayashi Y, Saigo K (2003) Resolution of 3-(methylamino)-1-(2-thienyl) propan-1-ol, a new key intermediate for duloxetine, with (S)-mandelic acid. Tetrahedron Asymm 14:1631–1636
Soni P, Kaul CL, Banerjee UC (2004) A novel process for the synthesis of a duloxetine intermediate, (S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)-1-propanamine. Indian patent application 1573/DEL/2004
Vallee B, Hoch F (1955) Zinc: a component of yeast alcohol dehydrogenase. Proc Natl Acad Sci USA 41:327–338
Wada M, Yoshizumi A, Furukawa Y, Kawabata H, Ueda M, Takagi H, Nakamori S (2004) Cloning and overexpression of the Exiguabacterium sp. F42 gene encoding a new short chain dehydrogenase, which catalyses the stereoselective reduction of ethyl 3-oxo-3-(2-thienyl)-propanoate to ethyl (S)-3-hydroxy-3-(2-thienyl)-propanoate. Biosci Biotechnol Biochem 68:1481–1488
Wong DT, Bymaster FP, Horng JS, Molly BB (1975) A new selective inhibitor for uptake of serotonin into synaptosomes of rat brain: 3-(p-trifluoromethylphenoxy). N-methyl-3-phenylpropylamine. J Pharmacol Exp Ther 193:804–811
Wong DT, Frazier J, Staten G, Staszak M, Weigel L (1990) Asymmetric synthesis and absolute stereochemistry of LY 248686. Tetrahedron Lett 31:7101–7104
Wong DT, Horng JS, Bymaster FP, Hauser KL, Molly BB (1974) A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci 15:471–479
Wong DT, Threlkeld PG, Best LL, Bymaster FP (1982) A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J Pharmacol Exp Ther 222:61–65
Acknowledgements
We are grateful to Dr. A.K. Chakraborati for helpful discussions. P.S. gratefully acknowledges the Council of Scientific and Industrial Research, India, for providing a Junior Research Fellowship
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soni, P., Banerjee, U.C. Biotransformations for the production of the chiral drug (S)-Duloxetine catalyzed by a novel isolate of Candida tropicalis. Appl Microbiol Biotechnol 67, 771–777 (2005). https://doi.org/10.1007/s00253-004-1870-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1870-5