Abstract.
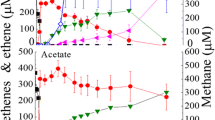
The pathway of methanol conversion by a thermophilic anaerobic consortium was elucidated by recording the fate of carbon in the presence and absence of bicarbonate and specific inhibitors. Results indicated that about 50% of methanol was directly converted to methane by the methylotrophic methanogens and 50% via the intermediates H2/CO2 and acetate. The deprivation of inorganic carbon species [Σ(HCO3 −+CO2)] in a phosphate-buffered system reduced the rate of methanol conversion. This suggests that bicarbonate is required as an electron (H2) sink and as a co-substrate for the efficient and complete removal of the chemical oxygen demand. Nuclear magnetic resonance spectroscopy was used to investigate the route of methanol conversion to acetate in bicarbonate-sufficient and bicarbonate-depleted environments. The proportions of [1,2-13C]acetate, [1-13C]acetate and [2-13C]acetate were determined. Methanol was preferentially incorporated into the methyl group of acetate, whereas HCO3 − was the preferred source of the carboxyl group. A small amount of the added H13CO3 − was reduced to form the methyl group of acetate and a small amount of the added 13CH3OH was oxidised and found in the carboxyl group of acetate when 13CH3OH was converted. The recovery of [13C]carboxyl groups in acetate from 13CH3OH was enhanced in bicarbonate-deprived medium. The small amount of label incorporated in the carboxyl group of acetate when 13CH3OH was converted in the presence of bromoethanesulfonic acid indicates that methanol can be oxidised to CO2 prior to acetate formation. These results indicate that methanol is converted through a common pathway (acetyl-CoA), being on the one hand reduced to the methyl group of acetate and on the other hand oxidised to CO2, with CO2 being incorporated into the carboxyl group of acetate.



Similar content being viewed by others
References
American PHA (1985) Standard methods for the examination of water and wastewater, 16th edn. American Public Health Association, Washington, D.C.
Balk M, Weijma J, Stams AJM (2002) Thermotoga lettingae sp. nov., a novel thermophilic, methanol degrading bacterium isolated from a thermophilic anaerobic reactor. Int J Syst Evol Microbiol 52:1361–1368
Cheng R (1977) Physical chemistry with applications to biological systems. Macmillan, New York
Conrad R, Klose M, Claus P (2000) Phosphate inhibits acetotrophic methanogenesis on rice roots. Appl Environ Microbiol 66:828–831
Davidova IA, Stams AJM (1996) Sulfate reduction with methanol by a thermophilic consortium obtained from a methanogenic reactor. Appl Microbiol Biotechnol 46:297–302
Florencio L, Field JA, Lettinga G (1994) Importance of cobalt for individual trophic groups in an anaerobic methanol-degrading consortium. Appl Environ Microbiol 60:227–234
Gonzalez-Gil G, Kleerebezem R, Lettinga G (1999) Effects of nickel and cobalt on kinetics of methanol conversion by methanogenic sludge as assessed by on-line CH4 monitoring. Appl Environ Microbiol 65:1789–1793
Hattori S, Kamagata Y, Hanada S, Shoun H (2000) Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int J Syst Evol Microbiol 50:1601–1609
Heijhuijsen JHFG, Hansen TA (1990) C1-Metabolism in anaerobic non-methanogenic bacteria. In: Codd GA, Dijkhuizen L, Tabita FR (eds) Autotrophic microbiology and one-carbon metabolism. Kluwer, Boston, pp 163–191
Kerby R, Niemczura W, Zeikus JG (1983) Single-carbon catabolism in acetogens: analysis of carbon flow in Acetobacterium woodii and Butyribacterium methylotrophicum by fermentation and 13C nuclear magnetic resonance measurement. J Bacteriol 155:1208–1218
Kim B-K, Daniels L (1991) Unexpected errors in gas chromatographic analysis of methane production by thermophilic bacteria. Appl Environ Microbiol 57:1866–1869
Kleerebezem R, Stams AJM (2000) Kinetics of syntrophic cultures: a theoretical treatise on butyrate fermentation. Biotechnol Bioeng 67:529–543
Lee MJ, Zinder SH (1988) Isolation and characterisation of a thermophilic bacterium which oxidises acetate in syntrophic association with a methanogen and which grows acetogenically on H2–CO2. Appl Environ Microbiol 54:124–129
Lettinga G, Van der Geest AT, Hobma S, Van der Laan J (1979) Anaerobic treatment of methanolic wastes. Water Res 13:725–737
Lettinga G, Zeeuw W de, Ouborg E (1981) Anaerobic treatment of wastes containing methanol and higher alcohols. Water Res 15:172–182
Ljungdahl LG (1986) The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol 40:415–450
Minami K, Horiyama T, Tasaki M, Tanimoto Y (1986) Methane production using a bio-reactor packed with pumice stone and evaporator condensate of a kraft pulp mill. J Ferment Technol 64:523–532
Minami K, Tanimoto Y, Tasaki M, Ogawa S, Okamura K (1988) Influence of sulphate on methane fermentation in a defined medium. Water Sci Technol 20:451–453
Minami K, Okamura K, Ogawa S, Naritomi T (1991) Continuous anaerobic treatment of wastewater from kraft pulp mill. J Ferment Bioeng 71:270–274
Pacaud S, Loubiere P, Goma G (1985) Methanol metabolism by Eubacterium limosum B2—effects of pH and carbon dioxide on growth and organic-acid production. Curr Microbiol 12:245–250
Paulo PL, Jiang B, Rebac S, Hulshoff-Pol L, Lettinga G (2001) Thermophilic anaerobic digestion of methanol in UASB reactor. Water Sci Technol 44:129–136
Paulo PL, Jiang B, Roest K, Lier JB van, Lettinga G (2002) Start-up of a thermophilic methanol-fed UASB reactor: change in sludge characteristics. Water Sci Technol 45:145–150
Schink B (1994) Diversity, ecology and isolation of acetogenic bacteria. In: Drake HL (ed) Acetogenesis. Chapman & Hall, New York, pp 197–235
Schink B, Zeikus JG (1980) Microbial methanol formation: a major end product of pectin metabolism. Curr Microbiol 4:387–390
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Van der Meijden P, Van der Drift C, Vogels GD (1984) Methanol conversion in Eubacterium-limosum. Arch Microbiol 138:360–364
Weijma J (2000) Methanol as electron donor for thermophilic biological sulphate and sulphite reduction. PhD thesis, Wageningen University, Wageningen
Weijma J, Stams AJM (2001) Methanol conversion in high-rate anaerobic reactors. Water Sci Technol 44:7–14
Weijma J, Stams A JM, Hulshoff Pol LW, Lettinga G (2000) Thermophilic sulfate reduction and methanogenesis with methanol in a high rate anaerobic reactor. Biotechnol Bioeng 67:354–363
Wood HG (1952) A study of carbon dioxide fixation by mass determination of the types of C13 acetate. J Biol Chem 194:905–931
Yamaguchi M, Hake J, Tanimoto Y, Naritomi T, Okamura K, Minami K (1991) Enzyme activity for monitoring the stability in a thermophilic anaerobic digestion of wastewater containing methanol. J Ferment Bioeng 71:264–269
Zinder SH (1990) Conversion of acetic acid to methane by thermophiles. Microb Rev 75:125–138
Zinder SH, Koch M (1984) Non-acetoclastic methanogenisis from acetate: acetate oxidation by a thermophilic syntrophic coculture. Arch Microbiol 138:263–272
Zinder SH, Anguish T, Cardwell SC (1984) Effects of temperature on methanogenesis in a thermophilic (58 °C) anaerobic digester. Appl Environ Microbiol 47:808–813
Acknowledgement.
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq project number 201055/97-0), an entity of the Brazilian Government for the development of science and technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paulo, P.L., Stams, A.J.M., Field, J.A. et al. Pathways of methanol conversion in a thermophilic anaerobic (55 °C) sludge consortium. Appl Microbiol Biotechnol 63, 307–314 (2003). https://doi.org/10.1007/s00253-003-1391-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1391-7